Page 256 - Instrumentation Reference Book 3E
P. 256
240 Temperature measurement
when absorbed by 1 kg of that substance, will
raise its temperature by 1 "C.
Specific heat capacity = J kg-'k-'
_--- -----
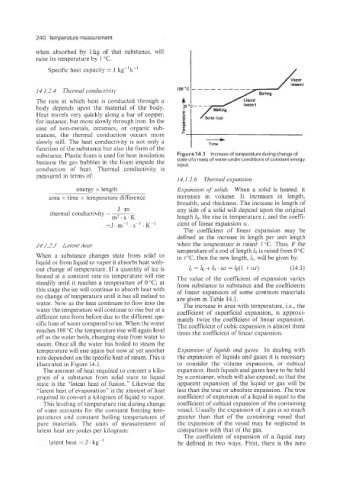
14.1.2.4 Tlzelmal conductivitli Boiling
The rate at which heat is conducted through a Liquid
(water)
body depends upon the material of the body. -/- Solid Melting (ice)
Heat travels very quickly along a bar of copper,
for instance, but more slowly through iron. In the
case of non-metals, ceramics, or organic sub- f
stances, the thermal conduction occurs more
slowly still. The heat conductivity is not only a ----b
Time
function of the substance but also the form of the
substance. Plastic foam is used for heat insulation Figure 14.1 Increase of temperature during change of
because the gas bubbles in the foam impede the state of a mass of water under conditions of constant energy
input.
conduction of heat. Thermal conductivity is
measured in terms of:
14.1.2.6 Thernial expansion
energy x length Expansion of solids When a solid is heated, it
area x time x temperature difference increases in volume. It increases in length,
breadth, and thickness. The increase in length of
J.m any side of a solid will depend upon the original
m2.s.K
thermal conductivity = ~ length lo, the rise in temperature t, and the coeffi-
=J . m-' . sP1 . K-' cient of linear expansion a.
The coefficient of linear expansion may be
defined as the increase in length per unit length
14.1.2.5 Latent heat when the temperature is raised 1 "C. Thus, if the
temperature of a rod of length 10 is raised from 0 "C
When a substance changes state from solid to to t"C, then the new length, lr, will be given by:
liquid or from liquid to vapor it absorbs heat with-
out change of temperature. If a quantity of ice is I, = lo + lo ' at = lo(1 +at) (14.1)
heated at a constant rate its temperature will rise The value of the coefficient of expansion varies
steadily until it reaches a temperature of 0°C; at from substance to substance and the coefficients
this stage the ice will continue to absorb heat with of linear expansion of some common materials
no change of temperature until it has all melted to are given in Table 14.1.
water. Now as the heat continues to flow into the The increase in area with temperature, Le., the
water the temperature will continue to rise but at a coefficient of superficial expansion, is approxi-
different rate from before due to the different spe- mately twice the coefficient of linear expansion.
cific heat of water compared to ice. When the water The coefficient of cubic expansion is almost three
reaches 100 "C the temperature rise will again level times the coefficient of linear expansion.
off as the water boils. changing state from water to
steam. Once all the water has boiled to steam the
temperature will rise again but now at yet another Expansion of liquids and gases In dealing with
rate dependent on the specific heat of steam. This is the expansion of liquids and gases it is necessary
illustrated in Figure 14.1. to consider the volume expansion, or cubical
The amount of heat required to convert a kilo- expansion. Both liquids and gases have to be held
gram of a substance from solid state to liquid by a container, which will also expand, so that the
state is the "latent heat of fusion." Likewise the apparent expansion of the liquid or gas will be
"latent heat of evaporation" is the amount of heat less than the true or absolute expansion. The true
required to convert a kilogram of liquid to vapor. coefficient of expansion of a liquid is equal to the
This leveling of temperature rise during change coefficient of cubical expansion of the containing
of state accounts for the constant freezing tem- vessel. Usually the expansion of a gas is so much
peratures and constant boiling temperatures of greater than that of the containing vessel that
pure materials. The units of measurement of the expansion of the vessel may be neglected in
latent heat are joules per kilogram: comparison with that of the gas.
The coefficient of expansion of a liquid may
latent heat = J . kg-' be defined in two ways. First, there is the zero