Page 105 - Materials Chemistry, Second Edition
P. 105
L1644_C03.fm Page 80 Tuesday, October 21, 2003 3:11 PM
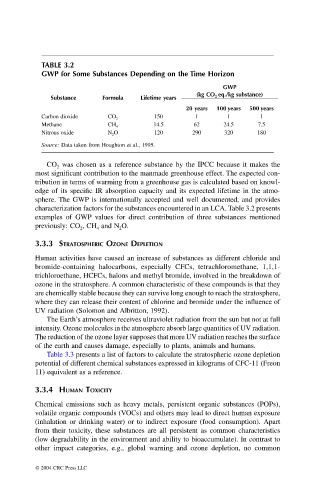
TABLE 3.2
GWP for Some Substances Depending on the Time Horizon
GWP
(kg CO 2 eq./kg substance)
Substance Formula Lifetime years
20 years 100 years 500 years
Carbon dioxide CO 2 150 1 1 1
Methane CH 4 14.5 62 24.5 7.5
Nitrous oxide N 2 O 120 290 320 180
Source: Data taken from Houghton et al., 1995.
CO was chosen as a reference substance by the IPCC because it makes the
2
most significant contribution to the manmade greenhouse effect. The expected con-
tribution in terms of warming from a greenhouse gas is calculated based on knowl-
edge of its specific IR absorption capacity and its expected lifetime in the atmo-
sphere. The GWP is internationally accepted and well documented, and provides
characterization factors for the substances encountered in an LCA. Table 3.2 presents
examples of GWP values for direct contribution of three substances mentioned
previously: CO , CH and N O.
2
4
2
3.3.3 STRATOSPHERIC OZONE DEPLETION
Human activities have caused an increase of substances as different chloride and
bromide-containing halocarbons, especially CFCs, tetrachloromethane, 1,1,1-
trichloroethane, HCFCs, halons and methyl bromide, involved in the breakdown of
ozone in the stratosphere. A common characteristic of these compounds is that they
are chemically stable because they can survive long enough to reach the stratosphere,
where they can release their content of chlorine and bromide under the influence of
UV radiation (Solomon and Albritton, 1992).
The Earth’s atmosphere receives ultraviolet radiation from the sun but not at full
intensity. Ozone molecules in the atmosphere absorb large quantities of UV radiation.
The reduction of the ozone layer supposes that more UV radiation reaches the surface
of the earth and causes damage, especially to plants, animals and humans.
Table 3.3 presents a list of factors to calculate the stratospheric ozone depletion
potential of different chemical substances expressed in kilograms of CFC-11 (Freon
11) equivalent as a reference.
3.3.4 HUMAN TOXICITY
Chemical emissions such as heavy metals, persistent organic substances (POPs),
volatile organic compounds (VOCs) and others may lead to direct human exposure
(inhalation or drinking water) or to indirect exposure (food consumption). Apart
from their toxicity, these substances are all persistent as common characteristics
(low degradability in the environment and ability to bioaccumulate). In contrast to
other impact categories, e.g., global warning and ozone depletion, no common
© 2004 CRC Press LLC