Page 185 - Intro Predictive Maintenance
P. 185
176 An Introduction to Predictive Maintenance
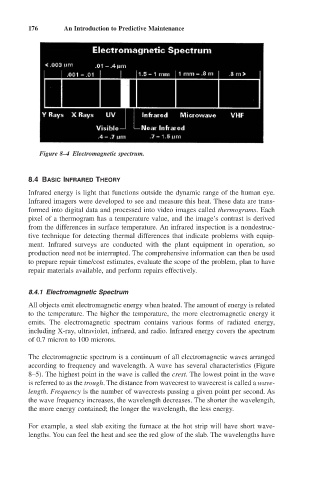
Figure 8–4 Electromagnetic spectrum.
8.4 BASIC INFRARED THEORY
Infrared energy is light that functions outside the dynamic range of the human eye.
Infrared imagers were developed to see and measure this heat. These data are trans-
formed into digital data and processed into video images called thermograms. Each
pixel of a thermogram has a temperature value, and the image’s contrast is derived
from the differences in surface temperature. An infrared inspection is a nondestruc-
tive technique for detecting thermal differences that indicate problems with equip-
ment. Infrared surveys are conducted with the plant equipment in operation, so
production need not be interrupted. The comprehensive information can then be used
to prepare repair time/cost estimates, evaluate the scope of the problem, plan to have
repair materials available, and perform repairs effectively.
8.4.1 Electromagnetic Spectrum
All objects emit electromagnetic energy when heated. The amount of energy is related
to the temperature. The higher the temperature, the more electromagnetic energy it
emits. The electromagnetic spectrum contains various forms of radiated energy,
including X-ray, ultraviolet, infrared, and radio. Infrared energy covers the spectrum
of 0.7 micron to 100 microns.
The electromagnetic spectrum is a continuum of all electromagnetic waves arranged
according to frequency and wavelength. A wave has several characteristics (Figure
8–5). The highest point in the wave is called the crest. The lowest point in the wave
is referred to as the trough. The distance from wavecrest to wavecrest is called a wave-
length. Frequency is the number of wavecrests passing a given point per second. As
the wave frequency increases, the wavelength decreases. The shorter the wavelength,
the more energy contained; the longer the wavelength, the less energy.
For example, a steel slab exiting the furnace at the hot strip will have short wave-
lengths. You can feel the heat and see the red glow of the slab. The wavelengths have