Page 263 - Introduction to Colloid and Surface Chemistry
P. 263
252 Rheology
viscosity and relative molecular mass can be expressed by the general
equation proposed by Mark and Houwink:
to] = KM? (9,6)
K and a are constants characteristic of the polymer-solvent system (a
depends on the configuration of the polymer chains) and approxim-
ately independent of relative molecular mass.
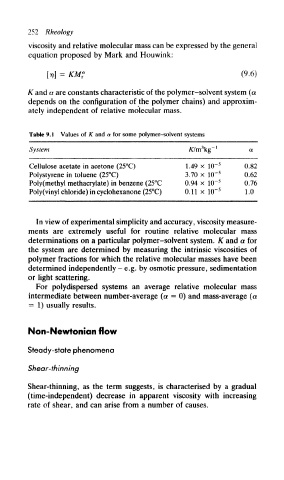
Table 9.1 Values of K and a for some polymer-solvent systems
System K/m\g~'
Cellulose acetate in acetone (25°C) 1.49 x 1(T 5 0.82
Polystyrene in toluene (25°C) 3.70 x 1(T 5 0.62
Poly(methyl methacrylate) in benzene (25°C 0.94 x 10~ 5 0.76
Poly(vinyl chloride) in cyclohexanone (25°C) 0.11 X HT 5 1.0
In view of experimental simplicity and accuracy, viscosity measure-
ments are extremely useful for routine relative molecular mass
determinations on a particular polymer-solvent system. K and a for
the system are determined by measuring the intrinsic viscosities of
polymer fractions for which the relative molecular masses have been
determined independently - e.g. by osmotic pressure, sedimentation
or light scattering.
For polydispersed systems an average relative molecular mass
intermediate between number-average (a = 0) and mass-average (a
= 1) usually results.
Non-Newtonian flow
Steady-state phenomena
Shear-thinning
Shear-thinning, as the term suggests, is characterised by a gradual
(time-independent) decrease in apparent viscosity with increasing
rate of shear, and can arise from a number of causes.