Page 94 - Introduction to Colloid and Surface Chemistry
P. 94
84 Liquid-gas and liquid-liquid interfaces
particularly useful when there is more than one surface-active species
or unavoidable surface-active impurity present. In such cases surface
tension measurements would probably be ambiguous.
Association colloids — micelle formation 48
Physical properties of surfactant solutions
Solutions of highly surface-active materials exhibit unusual physical
properties. In dilute solution the surfactant acts as a normal solute
(and in the case of ionic surfactants, normal electrolyte behaviour is
observed). At fairly well defined concentrations, however, abrupt
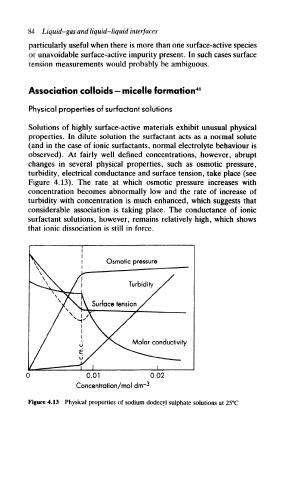
changes in several physical properties, such as osmotic pressure,
turbidity, electrical conductance and surface tension, take place (see
Figure 4.13). The rate at which osmotic pressure increases with
concentration becomes abnormally low and the rate of increase of
turbidity with concentration is much enhanced, which suggests that
considerable association is taking place. The conductance of ionic
surfactant solutions, however, remains relatively high, which shows
that ionic dissociation is still in force.
i Osmotic pressure
0.01 0.02
Concentration/mol dm~3
Figure 4.13 Physical properties of sodium dodecyl sulphate solutions at 25°C