Page 82 - Introduction to Petroleum Engineering
P. 82
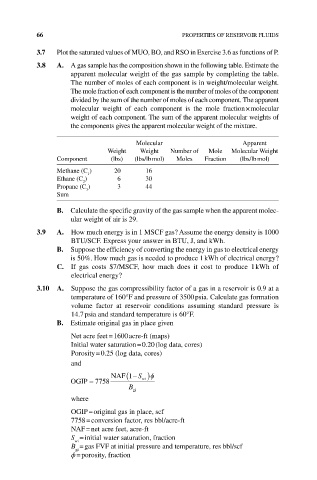
66 PROPERTIES OF RESERVOIR FLUIDS
3.7 Plot the saturated values of MUO, BO, and RSO in Exercise 3.6 as functions of P.
3.8 A. A gas sample has the composition shown in the following table. Estimate the
apparent molecular weight of the gas sample by completing the table.
The number of moles of each component is in weight/molecular weight.
The mole fraction of each component is the number of moles of the component
divided by the sum of the number of moles of each component. The apparent
molecular weight of each component is the mole fraction × molecular
weight of each component. The sum of the apparent molecular weights of
the components gives the apparent molecular weight of the mixture.
Molecular Apparent
Weight Weight Number of Mole Molecular Weight
Component (lbs) (lbs/lb mol) Moles Fraction (lbs/lb mol)
Methane (C ) 20 16
1
Ethane (C ) 6 30
2
Propane (C ) 3 44
3
Sum
b. Calculate the specific gravity of the gas sample when the apparent molec-
ular weight of air is 29.
3.9 A. How much energy is in 1 MSCF gas? Assume the energy density is 1000
BTU/SCF. Express your answer in BTU, J, and kWh.
b. Suppose the efficiency of converting the energy in gas to electrical energy
is 50%. How much gas is needed to produce 1 kWh of electrical energy?
C. If gas costs $7/MSCF, how much does it cost to produce 1 kWh of
electrical energy?
3.10 A. Suppose the gas compressibility factor of a gas in a reservoir is 0.9 at a
temperature of 160°F and pressure of 3500 psia. Calculate gas formation
volume factor at reservoir conditions assuming standard pressure is
14.7 psia and standard temperature is 60°F.
b. Estimate original gas in place given
Net acre feet = 1600 acre‐ft (maps)
Initial water saturation = 0.20 (log data, cores)
Porosity = 0.25 (log data, cores)
and
−
NAF(1 S wi )φ
OGIP = 7758
B
gi
where
OGIP = original gas in place, scf
7758 = conversion factor, res bbl/acre‐ft
NAF = net acre feet, acre‐ft
S = initial water saturation, fraction
wi
B = gas FVF at initial pressure and temperature, res bbl/scf
gi
ϕ = porosity, fraction