Page 13 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 13
2 Introduction to Transfer Phenomena in PEM Fuel Cells
1.1. Hydrogen as an energy vector
Hydrogen is the lightest and most abundant element in the universe.
Mixed with oxygen, it can burn by releasing energy. It has a large amount of
energy per unit mass; however, it contains a small amount of energy per unit
volume at room temperature and at atmospheric pressure.
Hydrogen is an energy vector virtually non-existent in nature at the
molecular level. This is why it must be produced by electrolysis, reforming
of vapors or natural gas, gasification of biomass, or by oxidation and
reforming of hydrocarbons or biomass. These methods are determined and
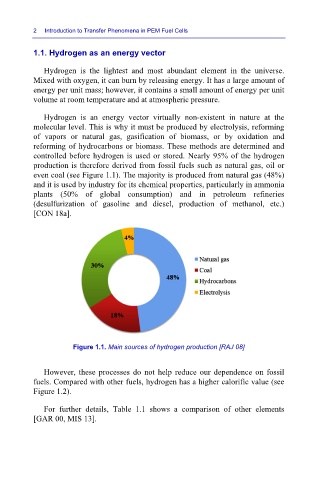
controlled before hydrogen is used or stored. Nearly 95% of the hydrogen
production is therefore derived from fossil fuels such as natural gas, oil or
even coal (see Figure 1.1). The majority is produced from natural gas (48%)
and it is used by industry for its chemical properties, particularly in ammonia
plants (50% of global consumption) and in petroleum refineries
(desulfurization of gasoline and diesel, production of methanol, etc.)
[CON 18a].
Figure 1.1. Main sources of hydrogen production [RAJ 08]
However, these processes do not help reduce our dependence on fossil
fuels. Compared with other fuels, hydrogen has a higher calorific value (see
Figure 1.2).
For further details, Table 1.1 shows a comparison of other elements
[GAR 00, MIS 13].