Page 143 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 143
132 Introduction to Transfer Phenomena in PEM Fuel Cells
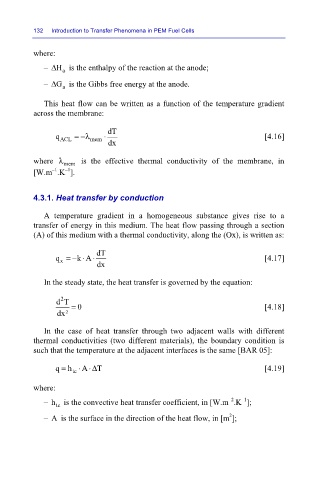
where:
– HΔ
– GΔ a a is the enthalpy of the reaction at the anode;
is the Gibbs free energy at the anode.
This heat flow can be written as a function of the temperature gradient
across the membrane:
dT
q ACL =−λ mem ⋅ [4.16]
dx
where λ mem is the effective thermal conductivity of the membrane, in
–1
–1
[W.m .K ].
4.3.1. Heat transfer by conduction
A temperature gradient in a homogeneous substance gives rise to a
transfer of energy in this medium. The heat flow passing through a section
(A) of this medium with a thermal conductivity, along the (Ox), is written as:
dT
q =− k A ⋅ [4.17]
⋅
x
dx
In the steady state, the heat transfer is governed by the equation:
2
dT = 0 [4.18]
dx²
In the case of heat transfer through two adjacent walls with different
thermal conductivities (two different materials), the boundary condition is
such that the temperature at the adjacent interfaces is the same [BAR 05]:
q = h ⋅ A⋅Δ T [4.19]
tc
where:
–2
–1
– h is the convective heat transfer coefficient, in [W.m .K ];
tc
2
– A is the surface in the direction of the heat flow, in [m ];