Page 169 - Introduction to chemical reaction engineering and kinetics
P. 169
6.7 Summary 151
E
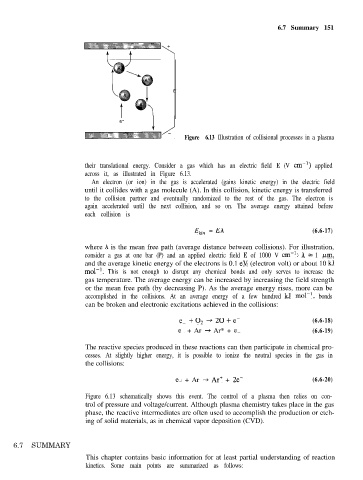
Figure 6.13 Illustration of collisional processes in a plasma
their translational energy. Consider a gas which has an electric field E (V cm-i) applied
across it, as illustrated in Figure 6.13.
An electron (or ion) in the gas is accelerated (gains kinetic energy) in the electric field
until it collides with a gas molecule (A). In this collision, kinetic energy is transferred
to the collision partner and eventually randomized to the rest of the gas. The electron is
again accelerated until the next collision, and so on. The average energy attained before
each collision is
Ekin = Eh (6.6-17)
where A is the mean free path (average distance between collisions). For illustration,
consider a gas at one bar (P) and an applied electric field E of 1000 V cm-‘: A = 1 pm,
and the average kinetic energy of the electrons is 0.1 eV (electron volt) or about 10 kJ
mol-l. This is not enough to disrupt any chemical bonds and only serves to increase the
gas temperature. The average energy can be increased by increasing the field strength
or the mean free path (by decreasing P). As the average energy rises, more can be
accomplished in the collisions. At an average energy of a few hundred kJ mol-l, bonds
can be broken and electronic excitations achieved in the collisions:
e- +Oo, + 20+e- (6.6-18)
e- + Ar + Ar* + e- (6.6-19)
The reactive species produced in these reactions can then participate in chemical pro-
cesses. At slightly higher energy, it is possible to ionize the neutral species in the gas in
the collisions:
e- + Ar -+ Ar+ + 2e- (6.6-20)
Figure 6.13 schematically shows this event. The control of a plasma then relies on con-
trol of pressure and voltage/current. Although plasma chemistry takes place in the gas
phase, the reactive intermediates are often used to accomplish the production or etch-
ing of solid materials, as in chemical vapor deposition (CVD).
6.7 SUMMARY
This chapter contains basic information for at least partial understanding of reaction
kinetics. Some main points are summarized as follows: