Page 231 - Introduction to chemical reaction engineering and kinetics
P. 231
8.5 Heterogeneous Catalysis: Kinetics in Porous Catalyst Particles 213
) 5.0 10.0 50 100 500 1000
4
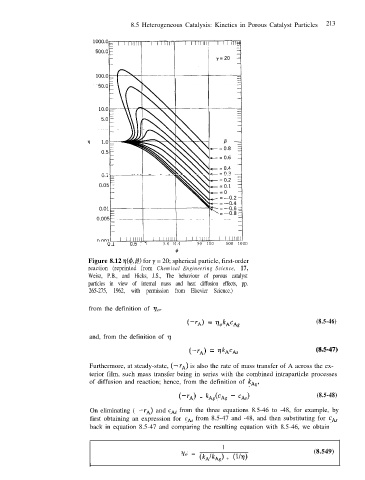
Figure 8.12 ~(4, p) for y = 20; spherical particle, first-order
reaction (reprinted from Chemical Engineering Science, 17,
Weisz, P.B., and Hicks, J.S., The behaviour of porous catalyst
particles in view of internal mass and heat diffusion effects, pp.
265-275, 1962, with permission from Elsevier Science.)
from the definition of T,I~,
(8.5-46)
Cer*) = V&ACA~
and, from the definition of 7
(8.547)
C-~A) = T~ACA,
Furthermore, at steady-state, (- rA) is also the rate of mass transfer of A across the ex-
terior film, such mass transfer being in series with the combined intraparticle processes
of diffusion and reaction; hence, from the definition of k&,
(8.5-48)
(-rA) = kAg(cAg - cAs)
On eliminating ( -rA) and cllr from the three equations 8.5-46 to -48, for example, by
first obtaining an expression for cAs from 8.5-47 and -48, and then substituting for cAs
back in equation 8.5-47 and comparing the resulting equation with 8.5-46, we obtain
1
70 = (8.549)
(k~/kAg) + (l/T)
I /