Page 46 - Introduction to chemical reaction engineering and kinetics
P. 46
28 Chapter 2: Kinetics and Ideal Reactor Models
Then equation 2.2-2 becomes, for i = A,
(-rA) = -(v,lV)(dSldt) (2.2-6) /
(3) Normalization may be by means of the system volume V . This converts nA into
a volumetric molar concentration (molarity) of A, CA, defined by
If we replace nA in equation 2.2-2 by cAV and allow V to vary, then we have
(-).A) = 2!2$ - ?$ (2.2-8)
Since (-?-A) is now related to two quantities, CA and V, we require additional
information connecting CA (or nA) and V. This is provided by an equation of
state of the general form
v = v(nA, T, P)
(3a) A special case of equation 2.2-8 results if the reacting system has constant vol-
ume (i.e., is of constant density). Then dVldt = 0, and
(-,-A) = -dc,/dt (constant density) (2.2-10)
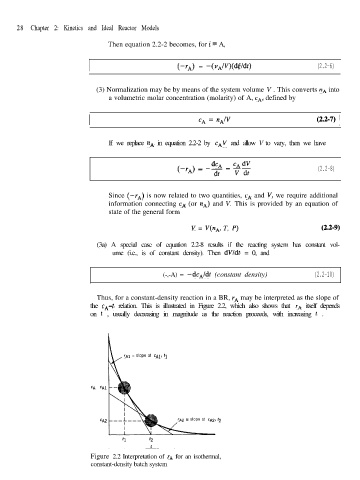
Thus, for a constant-density reaction in a BR, r, may be interpreted as the slope of
the CA-t relation. This is illustrated in Figure 2.2, which also shows that rA itself depends
on t , usually decreasing in magnitude as the reaction proceeds, with increasing t .
rAl = slope at cA1, tl
rA2 = slope at cA2, tp
Figure 2.2 Interpretation of rA for an isothermal,
constant-density batch system