Page 27 - Lignocellulosic Biomass to Liquid Biofuels
P. 27
Fundamentals of lignocellulosic biomass 7
type of biomass is abundant and cheap. They can be collected from a
wide range of sources, such as
• agriculture field wastes (paddy husks, straw, grasses, crop stubble, and
trash) and
• agricultural processing wastes (palm oil waste, sugarcane bagasse and
animal paunch waste, cotton gin trash, etc.).
1.4 Chemistry of lignocellulosic biomass
Before studying the biorefinery of LCB, it is essential to understand the
chemistry of individual biomass because the structure or composition of
biomass affects the chemical pretreatment, enzymatic digestibility, and the
generation of compounds inhibiting fermentative microorganisms used to
produce the final fuel or chemical. Chemical or spectroscopic analysis can
determine the percentages of individual sugars, protein, uronic acids, and
lignin. For example, during the conversion of corn stover, hardwoods, or
rice straw, we are in fact working primarily with the plant’s structural
parts, most of which are cell walls. Therefore more knowledge is required
about the natural composition and structure of polymers and chemicals in
plant tissue.
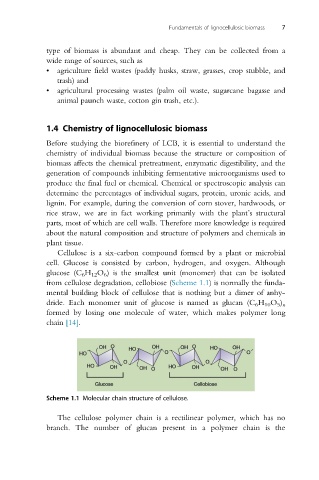
Cellulose is a six-carbon compound formed by a plant or microbial
cell. Glucose is consisted by carbon, hydrogen, and oxygen. Although
glucose (C 6 H 12 O 6 ) is the smallest unit (monomer) that can be isolated
from cellulose degradation, cellobiose (Scheme 1.1) is normally the funda-
mental building block of cellulose that is nothing but a dimer of anhy-
dride. Each monomer unit of glucose is named as glucan (C 6 H 10 O 5 ) n
formed by losing one molecule of water, which makes polymer long
chain [14].
Scheme 1.1 Molecular chain structure of cellulose.
The cellulose polymer chain is a rectilinear polymer, which has no
branch. The number of glucan present in a polymer chain is the