Page 274 - Lignocellulosic Biomass to Liquid Biofuels
P. 274
230 Lignocellulosic Biomass to Liquid Biofuels
data set, and periodic standards are required to check the variations. Also
process parameters such as velocity, temperature, CO, and H 2 reactor
pressure require investigations to develop kinetic models.
A kinetic model was developed by Kellner and Bell [111] and
Takoudis [112] for the hydrocarbons production without assumptions on
a rate-determining step. However, several assumptions were introduced to
solve the resulting set of equations. In general, the development of kinetic
model should be based on rate-determining steps with particular mecha-
nistic scheme during FT synthesis.
The FT synthesis could be expressed as the combination of the FT
reaction and the WGS reaction [Eqs. (7.6) and (7.7)].
m 1
CO 1 1 1 H 2 - C n H m 1 H 2 O R FT Þ (7.6)
ð
2n n
CO 1 H 2 O2CO 2 1 H 2 R WGS Þ (7.7)
ð
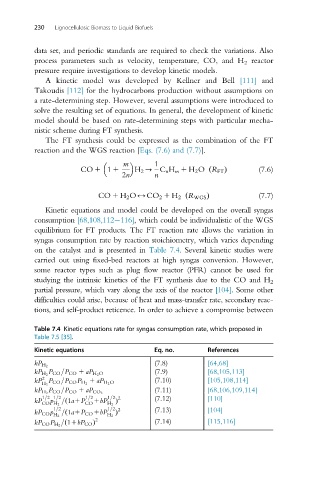
Kinetic equations and model could be developed on the overall syngas
consumption [68,108,112 116], which could be individualistic of the WGS
equilibrium for FT products. The FT reaction rate allows the variation in
syngas consumption rate by reaction stoichiometry, which varies depending
on the catalyst and is presented in Table 7.4. Several kinetic studies were
carried out using fixed-bed reactors at high syngas conversion. However,
some reactor types such as plug flow reactor (PFR) cannot be used for
studying the intrinsic kinetics of the FT synthesis due to the CO and H 2
partial pressure, which vary along the axis of the reactor [104].Someother
difficulties could arise, because of heat and mass-transfer rate, secondary reac-
tions, and self-product reticence. In order to achieve a compromise between
Table 7.4 Kinetic equations rate for syngas consumption rate, which proposed in
Table 7.5 [35].
Kinetic equations Eq. no. References
(7.8) [64,68]
kP H 2
P (7.9) [68,105,113]
kP H 2 CO =P CO 1 aP H 2 O
2
kP P CO =P CO P H 2 1 aP H 2 O (7.10) [105,108,114]
H 2
P (7.11) [68,106,109,114]
kP H 2 CO =P CO 1 aP CO 2
1=2 1=2 1=2 1=2 2 (7.12) [110]
kP p =ð1a1P 1bP Þ
CO H 2 CO H 2
1=2 1=2 2 (7.13) [104]
kP CO p =ð1a1P CO 1bP Þ
H 2 H 2
2
=ð11bP CO Þ (7.14) [115,116]
kP CO P H 2