Page 149 - Lindens Handbook of Batteries
P. 149
6.2 PRINCIPLES OF OPERATION
100
Model
90 Data
80
Discharge capacity (Ah) 70
60
50
Current at 0.05 C rate
1
Discharge time =
40
Discharge current F 0.05
30
20
0 50 100 150 200 250 300 350 400 450 500
Discharge current (A)
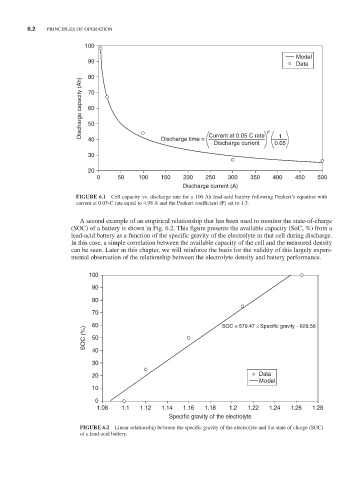
FiguRE 6.1 Cell capacity vs. discharge rate for a 100 Ah lead-acid battery following Peukert’s equation with
current at 0.05-C rate equal to 4.98 A and the Peukert coefficient (F) set to 1.3.
A second example of an empirical relationship that has been used to monitor the state-of-charge
(SOC) of a battery is shown in Fig. 6.2. This figure presents the available capacity (SoC, %) from a
lead-acid battery as a function of the specific gravity of the electrolyte in that cell during discharge.
In this case, a simple correlation between the available capacity of the cell and the measured density
can be seen. Later in this chapter, we will reinforce the basis for the validity of this largely experi-
mental observation of the relationship between the electrolyte density and battery performance.
100
90
80
70
60 SOC = 579.47 × Specific gravity – 628.56
SOC (%) 50
40
30
20 Data
Model
10
0
1.08 1.1 1.12 1.14 1.16 1.18 1.2 1.22 1.24 1.26 1.28
Specific gravity of the electrolyte
FiguRE 6.2 Linear relationship between the specific gravity of the electrolyte and the state of charge (SOC)
of a lead-acid battery.