Page 162 - Lindens Handbook of Batteries
P. 162
MATHEMATICAL MODELING OF BATTERIES 6.15
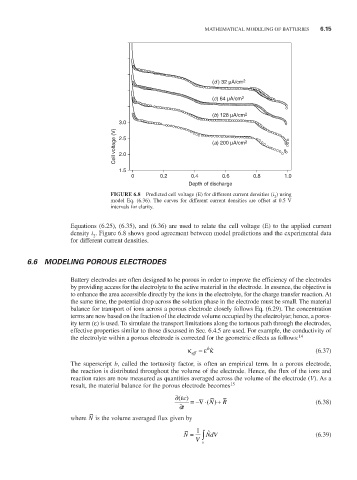
(d ) 32 µA/cm 2
(c) 64 µA/cm 2
(b) 128 µA/cm 2
3.0
Cell voltage (V) 2.5 (a) 200 µA/cm 2
2.0
1.5
0 0.2 0.4 0.6 0.8 1.0
Depth of discharge
FiguRE 6.8 Predicted cell voltage (E) for different current densities (i ) using
2
model Eq. (6.36). The curves for different current densities are offset at 0.5 V
intervals for clarity.
Equations (6.25), (6.35), and (6.36) are used to relate the cell voltage (E) to the applied current
density i . Figure 6.8 shows good agreement between model predictions and the experimental data
2
for different current densities.
6.6 MODELING POROUS ELECTRODES
Battery electrodes are often designed to be porous in order to improve the efficiency of the electrodes
by providing access for the electrolyte to the active material in the electrode. In essence, the objective is
to enhance the area accessible directly by the ions in the electrolyte, for the charge transfer reaction. At
the same time, the potential drop across the solution phase in the electrode must be small. The material
balance for transport of ions across a porous electrode closely follows Eq. (6.29). The concentration
terms are now based on the fraction of the electrode volume occupied by the electrolyte; hence, a poros-
ity term (ε) is used. To simulate the transport limitations along the tortuous path through the electrodes,
effective properties similar to those discussed in Sec. 6.4.5 are used. For example, the conductivity of
the electrolyte within a porous electrode is corrected for the geometric effects as follows: 14
κ eff = ε b κ ˆ (6.37)
The superscript b, called the tortuosity factor, is often an empirical term. In a porous electrode,
the reaction is distributed throughout the volume of the electrode. Hence, the flux of the ions and
reaction rates are now measured as quantities averaged across the volume of the electrode (V). As a
result, the material balance for the porous electrode becomes 15
∂()εc
+
N
=-∇⋅() R (6.38)
∂t
where N is the volume averaged flux given by
1
ˆ
N = ∫ NdV (6.39)
V v