Page 177 - Lindens Handbook of Batteries
P. 177
7.2 PRINCIPLES OF OPERATION
bases cause lower pH values. For example, in the Leclanché electrolyte, zinc chloride is an important
electrolyte component, and its complex equilibria in aqueous media shift the pH to mildly acidic
conditions. Likewise, the ubiquitous presence of carbon dioxide dissolved in aqueous solutions shifts
the pH to mild-acid conditions. It must always be borne in mind that the equilibrium voltage window
for aqueous solutions is approximately 1.2 V (the actual value depends on electrolyte concentration,
temperature and other factors) so that gassing of hydrogen with anodes more active than hydrogen
and of oxygen for cathodes more active than oxygen will occur to some extent even in the presence
of passive films on the electrodes. Thus, much of the electrolyte work in aqueous media deals with
the twin problems of anode corrosion and cathode gassing and the means to control these deleterious
reactions. The situation with rechargeable batteries is even more stringent because of the increased
potential of both negative and positive electrodes during charge.
7.2.1 Alkaline Electrolytes
Alkaline electrolytes are utilized in a large variety of primary and rechargeable batteries. The most
commonly used primary battery type is the so-called alkaline manganese-dioxide battery, described
in Chap. 11 of this volume. Other primary batteries with alkaline electrolytes are the various button
cells such as zinc-silver oxide and zinc-air (Chap. 13), and zinc-mercuric oxide, which are made
in both button and cylindrical format (Chap. 12). Rechargeable cells using alkaline electrolytes
include nickel-metal hydride (Chap. 22), nickel-cadmium (Chaps. 19–21), nickel-zinc (Chap. 23),
and nickel-hydrogen (Chap. 24).
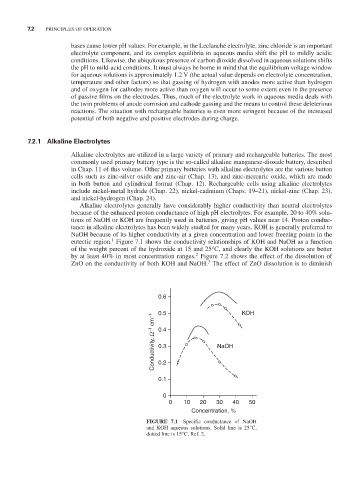
Alkaline electrolytes generally have considerably higher conductivity than neutral electrolytes
because of the enhanced proton conductance of high pH electrolytes. For example, 20 to 40% solu-
tions of NaOH or KOH are frequently used in batteries, giving pH values near 14. Proton conduc-
tance in alkaline electrolytes has been widely studied for many years. KOH is generally preferred to
NaOH because of its higher conductivity at a given concentration and lower freezing points in the
1
eutectic region. Figure 7.1 shows the conductivity relationships of KOH and NaOH as a function
of the weight percent of the hydroxide at 15 and 25°C, and clearly the KOH solutions are better
2
by at least 40% in most concentration ranges. Figure 7.2 shows the effect of the dissolution of
3
ZnO on the conductivity of both KOH and NaOH. The effect of ZnO dissolution is to diminish
0.6
0.5 KOH
Conductivity, Ω –1 cm –1 0.4 NaOH
0.3
0.2
0.1
0
0 10 20 30 40 50
Concentration, %
FIGURE 7.1 Specific conductance of NaOH
and KOH aqueous solutions. Solid line is 25°C,
dotted line is 15°C, Ref. 2.