Page 185 - Lindens Handbook of Batteries
P. 185
7.10 PRINCIPLES OF OPERATION
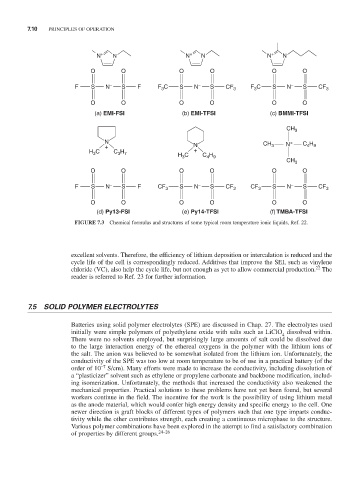
N + N N + N N + N
O O O O O O
F S N – S F F C S N – S CF 3 F C S N – S CF 3
3
3
O O O O O O
(a) EMI-FSI (b) EMI-TFSI (c) BMMI-TFSI
CH 3
N N CH N + C H
+ N 3 4 9
H 3 C C H +
3 7
4 9
H 3 C C H
CH 3
O O O O O O
F S N – S F CF 3 S N – S CF 3 CF 3 S N – S CF 3
O O O O O O
(d) Py13-FSI (e) Py14-TFSI (f) TMBA-TFSI
FIGURE 7.3 Chemical formulas and structures of some typical room temperature ionic liquids, Ref. 22.
excellent solvents. Therefore, the efficiency of lithium deposition or intercalation is reduced and the
cycle life of the cell is correspondingly reduced. Additives that improve the SEI, such as vinylene
22
chloride (VC), also help the cycle life, but not enough as yet to allow commercial production. The
reader is referred to Ref. 23 for further information.
7.5 sOlID POlymeR eleCTROlyTes
Batteries using solid polymer electrolytes (SPE) are discussed in Chap. 27. The electrolytes used
initially were simple polymers of polyethylene oxide with salts such as LiClO dissolved within.
4
There were no solvents employed, but surprisingly large amounts of salt could be dissolved due
to the large interaction energy of the ethereal oxygens in the polymer with the lithium ions of
the salt. The anion was believed to be somewhat isolated from the lithium ion. Unfortunately, the
conductivity of the SPE was too low at room temperature to be of use in a practical battery (of the
order of 10 S/cm). Many efforts were made to increase the conductivity, including dissolution of
-7
a “plasticizer” solvent such as ethylene or propylene carbonate and backbone modification, includ-
ing isomerization. Unfortunately, the methods that increased the conductivity also weakened the
mechanical properties. Practical solutions to these problems have not yet been found, but several
workers continue in the field. The incentive for the work is the possibility of using lithium metal
as the anode material, which would confer high energy density and specific energy to the cell. One
newer direction is graft blocks of different types of polymers such that one type imparts conduc-
tivity while the other contributes strength, each creating a continuous microphase to the structure.
Various polymer combinations have been explored in the attempt to find a satisfactory combination
of properties by different groups. 24–26