Page 52 - Lindens Handbook of Batteries
P. 52
ELECTROCHEMICAL PRINCIPLES AND REACTIONS 2.9
The exchange current as defined in Eq. (2.15) is a parameter of interest to researchers in the
battery field. This parameter may be conveniently expressed in terms of the rate constant k by com-
bining Eqs. (2.10), (2.12), (2.17), and (2.20),
α
α
i = nFAkC O (1 - ) C (2.21)
R
o
The exchange current i is a measure of the rate of exchange of charge between oxidized and reduced
o
species at any equilibrium potential without net overall change. The rate constant k, however, has
been defined for a particular potential, the formal standard potential of the system. It is not in
itself sufficient to characterize the system unless the transfer coefficient is also known. However,
Eq. (2.21) can be used in the elucidation of the electrode reaction mechanism. The value of the
transfer coefficient can be determined by measuring the exchange current density as a function of
the concentration of the reduction or oxidation species at a constant concentration of the oxidation
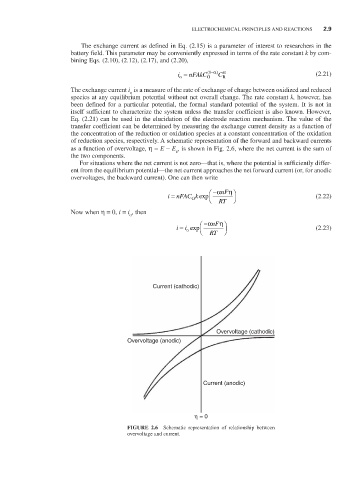
of reduction species, respectively. A schematic representation of the forward and backward currents
as a function of overvoltage, η = E - E , is shown in Fig. 2.6, where the net current is the sum of
e
the two components.
For situations where the net current is not zero—that is, where the potential is sufficiently differ-
ent from the equilibrium potential—the net current approaches the net forward current (or, for anodic
overvoltages, the backward current). One can then write
nF
i = nFAC k exp - α RT η (2.22)
O
Now when η = 0, i = i , then
o
nF
i = i exp - α RT η (2.23)
o
Current (cathodic)
Overvoltage (cathodic)
Overvoltage (anodic)
Current (anodic)
η = 0
FIGURE 2.6 Schematic representation of relationship between
overvoltage and current.