Page 183 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 183
|62 Chapter 6 Nonferrous Metals and Alloys: Production, General Properties, and Applications
6.7 Titanium and Titanium Alloys
Titanium (Ti, named after the Greek god Titan) is a silvery white metal discovered in
1791, but not produced commercially until the 1950s. Although titanium is
expensive, its high strength-to-weight ratio and corrosion resistance at room and
elevated temperatures make it attractive for many applications, including aircraft; jet
engines (see Fig. 6.1); racing cars; golf clubs; chemical, petrochemical, and marine
components; submarine hulls; armor plate; and medical applications, such as orthope-
dic implants (Table 6.10). Titanium alloys have been developed for service at 550°C
for long periods of time and at up to 75 0°C for shorter periods.
Unalloyed titanium, known as commercially pure titanium, has excellent
corrosion resistance for applications where strength considerations are secondary.
Aluminum, vanadium, molybdenum, manganese, and other alloying elements impart
properties such as improved workability, strength, and hardenability.
The properties and manufacturing characteristics of titanium alloys are ex-
tremely sensitive to small variations in both alloying and residual elements. Therefore,
control of composition and processing are important, especially the prevention of
surface contamination by hydrogen, oxygen, or nitrogen during processing; these
elements cause embrittlement of titanium and, consequently, reduce toughness and
ductility.
The body-centered cubic structure of titanium (beta-titanium) is above 880°C
and is ductile, whereas its hexagonal close-packed structure (alpha-titanium) is some-
what brittle and is very sensitive to stress corrosion. A variety of other structures
(alpha, near-alpha, alpha-beta, and beta) can be obtained by alloying and heat treat-
ing, so that the properties can be optimized for specific applications. Titanium alu-
minide intermetallics (TiAl and Ti3Al; see Section 4.2.2) have higher stiffness and
lower density than conventional titanium alloys and can withstand higher tempera-
tures.
Production. Ores containing titanium first are reduced to titanium tetrachloride in
an arc furnace, then converted to titanium chloride in a chlorine atmosphere. This
compound is reduced further to titanium metal by distillation and leaching (dissolv-
ing). This sequence forms sponge titanium, which is then pressed into billets, melted,
and poured into ingots to be processed later into various shapes. The complexity of
these multistep thermochemical operations (the Kroll process developed in the
1940-1950s) adds considerably to the cost of titanium. New developments in elec-
trochemical extraction processes are taking place to reduce the number of steps
involved and the energy consumption, thereby reducing the cost of producing
titanium.
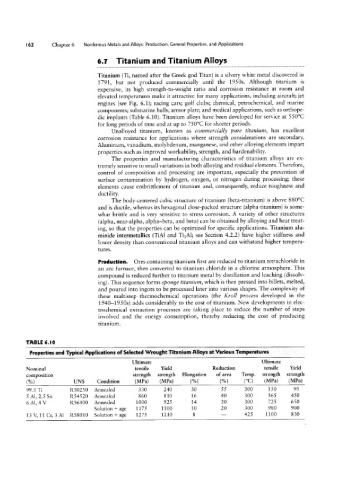
TABLE 6. I 0
Properties and Typical Applications of Selected Wrought Titanium Alloys at Various Temperatures
Ultimate Ultimate
Nominal tensile Yield Reduction tensile Yield
composition strength strength Elongation of area Temp. strength strength
(%) UNS Condition (MPa) (MPa) (°C) (MPa) (MPa)
99.5 Ti R50250 Annealed 330 240 300 150 95
5 Al, 2.5 Sn R54520 Annealed 860 810 300 565 450
6 Al, 4 V R56400 Annealed 1000 925 300 725 650
Solution + age 1175 1100 300 980 900
13 V, 11 Cr, 3 Al R58010 Solution + age 1275 1210 425 1100 830