Page 194 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 194
Section 7.2 The Structure of Polymers
H ill s * ` i f if C
Plasticizers
Stabilizers 3
Colorants ""} I
ren
Flame retardants
Lubricants
_ _ gg ` i t'i "i" ` ` ' : I _ Thermop|astics:Acrylics, ABS, nylons
Heat, PV€‘SSUfe»
polycarbonates, polyethylenes
polyvinyl chloride etc
Kjéfgr `ii
mars
Polymer
<rrri” “ ~
Catalyst
Thermosets:Epoxies,phenolics
ee <rr»
polyimides, etc.
i :
' |
|:>O|ymeliZatiOn; Amoréhous E Elastomers: Natural and synthetic rubbers
Condengatign, Pamy Crystamne : silicones, polyurethanes etc
a°ld'l'°“ Linear Cross-linking
Branched
Homopolymer
Copolymer
Terpolymer
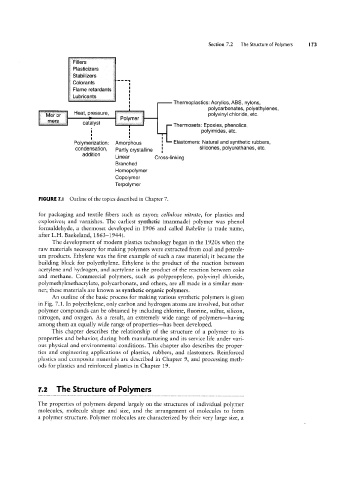
FIGURE 1.l Outline of the topics described in Chapter 7.
for packaging and textile fibers such as rayon; cellulose nitrate, for plastics and
explosives; and varnishes. The earliest synthetic (manmade) polymer was phenol
formaldehyde, a thermoset developed in 1906 and called Bakelite (a trade name,
after L.H. Baekeland, 1863-1944).
The development of modern plastics technology began in the 1920s when the
raw materials necessary for making polymers were extracted from coal and petrole-
um products. Ethylene was the first example of such a raw material; it became the
building block for polyethylene. Ethylene is the product of the reaction between
acetylene and hydrogen, and acetylene is the product of the reaction between coke
and methane. Commercial polymers, such as polypropylene, polyvinyl chloride,
polymethylmethacrylate, polycarbonate, and others, are all made in a similar man-
ner; these materials are known as synthetic organic polymers.
An outline of the basic process for making various synthetic polymers is given
in Fig. 7.1. In polyethylene, only carbon and hydrogen atoms are involved, but other
polymer compounds can be obtained by including chlorine, fluorine, sulfur, silicon,
nitrogen, and oxygen. As a result, an extremely wide range of polymers--having
among them an equally wide range of properties-has been developed.
This chapter describes the relationship of the structure of a polymer to its
properties and behavior, during both manufacturing and its service life under vari-
ous physical and environmental conditions. This chapter also describes the proper-
ties and engineering applications of plastics, rubbers, and elastomers. Reinforced
plastics and composite materials are described in Chapter 9, and processing meth-
ods for plastics and reinforced plastics in Chapter 19.
7.2 The Structure of Polymers
The properties of polymers depend largely on the structures of individual polymer
molecules, molecule shape and size, and the arrangement of molecules to form
a polymer structure. Polymer molecules are characterized by their very large size, a