Page 15 - Materials Chemistry, Second Edition
P. 15
2 1 What Is Materials Chemistry?
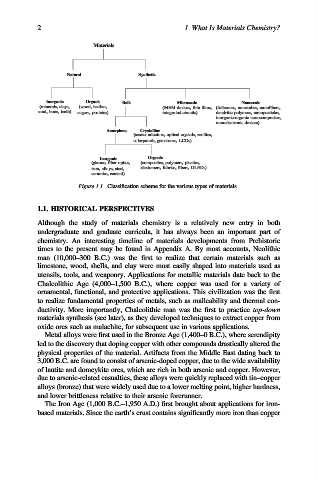
Materials
Natural
Synthetic
Inorganic Organic
Nanoscale
Bulk Microscale
(minerals, clays, (wood, leather, (MEM devices, thin films, (fullerenes, nanotubes, nanofibers,
sand, bone, teeth) sugars, proteins) integrated circuits) dendritic polymers, nanoparticles,
inorganic-organic nanocomposites,
nanoelectronic devices)
Amorphous Crystalline
(semiconductors, optical crystals, zeolites,
solarpanels, gemstones, LCDs)
Organic
Inorganic
(glasses, fiber optics, (composites, polymers, plastics,
iron, alloys, steel, elastomers, fabrics, fibers, OLEDs)
ceramics, cement)
Figure 1.1. Classification scheme for the various types of materials.
1.1. HISTORICAL PERSPECTIVES
Although the study of materials chemistry is a relatively new entry in both
undergraduate and graduate curricula, it has always been an important part of
chemistry. An interesting timeline of materials developments from Prehistoric
times to the present may be found in Appendix A. By most accounts, Neolithic
man (10,000–300 B.C.) was the first to realize that certain materials such as
limestone, wood, shells, and clay were most easily shaped into materials used as
utensils, tools, and weaponry. Applications for metallic materials date back to the
Chalcolithic Age (4,000–1,500 B.C.), where copper was used for a variety of
ornamental, functional, and protective applications. This civilization was the first
to realize fundamental properties of metals, such as malleability and thermal con-
ductivity. More importantly, Chalcolithic man was the first to practice top-down
materials synthesis (see later), as they developed techniques to extract copper from
oxide ores such as malachite, for subsequent use in various applications.
Metal alloys were first used in the Bronze Age (1,400–0 B.C.), where serendipity
led to the discovery that doping copper with other compounds drastically altered the
physical properties of the material. Artifacts from the Middle East dating back to
3,000 B.C. are found to consist of arsenic-doped copper, due to the wide availability
of lautite and domeykite ores, which are rich in both arsenic and copper. However,
due to arsenic-related casualties, these alloys were quickly replaced with tin–copper
alloys (bronze) that were widely used due to a lower melting point, higher hardness,
and lower brittleness relative to their arsenic forerunner.
The Iron Age (1,000 B.C.–1,950 A.D.) first brought about applications for iron-
based materials. Since the earth’s crust contains significantly more iron than copper