Page 117 - Mechanical Engineers' Handbook (Volume 4)
P. 117
106 Thermodynamics Fundamentals
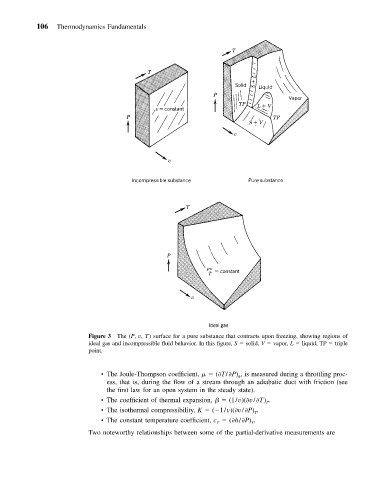
Figure 3 The (P, v, T) surface for a pure substance that contracts upon freezing, showing regions of
ideal gas and incompressible fluid behavior. In this figure, S solid, V vapor, L liquid, TP triple
point.
• The Joule-Thompson coefficient,
( T/ P) , is measured during a throttling proc-
h
ess, that is, during the flow of a stream through an adiabatic duct with friction (see
the first law for an open system in the steady state).
• The coefficient of thermal expansion, (1/v)( v/ T) .
P
• The isothermal compressibility, K ( 1/v)( v/ P) .
T
• The constant temperature coefficient, c ( h/ P) .
T
T
Two noteworthy relationships between some of the partial-derivative measurements are