Page 539 - Mechanical Engineers' Handbook (Volume 4)
P. 539
528 Cryogenic Systems
42
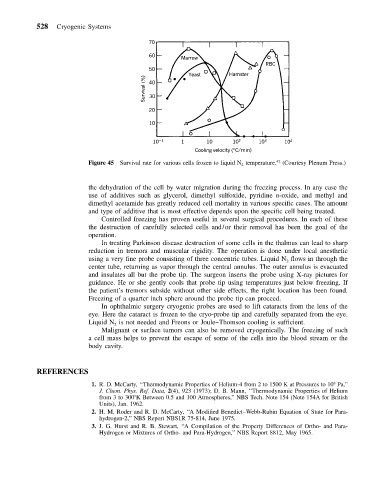
Figure 45 Survival rate for various cells frozen to liquid N 2 temperature. (Courtesy Plenum Press.)
the dehydration of the cell by water migration during the freezing process. In any case the
use of additives such as glycerol, dimethyl sulfoxide, pyridine n-oxide, and methyl and
dimethyl acetamide has greatly reduced cell mortality in various specific cases. The amount
and type of additive that is most effective depends upon the specific cell being treated.
Controlled freezing has proven useful in several surgical procedures. In each of these
the destruction of carefully selected cells and/or their removal has been the goal of the
operation.
In treating Parkinson disease destruction of some cells in the thalmus can lead to sharp
reduction in tremors and muscular rigidity. The operation is done under local anesthetic
using a very fine probe consisting of three concentric tubes. Liquid N flows in through the
2
center tube, returning as vapor through the central annulus. The outer annulus is evacuated
and insulates all but the probe tip. The surgeon inserts the probe using X-ray pictures for
guidance. He or she gently cools that probe tip using temperatures just below freezing. If
the patient’s tremors subside without other side effects, the right location has been found.
Freezing of a quarter inch sphere around the probe tip can proceed.
In ophthalmic surgery cryogenic probes are used to lift cataracts from the lens of the
eye. Here the cataract is frozen to the cryo-probe tip and carefully separated from the eye.
Liquid N is not needed and Freons or Joule–Thomson cooling is sufficient.
2
Malignant or surface tumors can also be removed cryogenically. The freezing of such
a cell mass helps to prevent the escape of some of the cells into the blood stream or the
body cavity.
REFERENCES
8
1. R. D. McCarty, ‘‘Thermodynamic Properties of Helium-4 from 2 to 1500 K at Pressures to 10 Pa,’’
J. Chem. Phys. Ref. Data, 2(4), 923 (1973); D. B. Mann, ‘‘Thermodynamic Properties of Helium
from 3 to 300 K Between 0.5 and 100 Atmospheres,’’ NBS Tech. Note 154 (Note 154A for British
Units), Jan. 1962.
2. H. M. Roder and R. D. McCarty, ‘‘A Modified Benedict–Webb-Rubin Equation of State for Para-
hydrogen-2,’’ NBS Report NBS1R 75-814, June 1975.
3. J. G. Hurst and R. B. Stewart, ‘‘A Compilation of the Property Differences of Ortho- and Para-
Hydrogen or Mixtures of Ortho- and Para-Hydrogen,’’ NBS Report 8812, May 1965.