Page 131 - Mechanism and Theory in Organic Chemistry
P. 131
Appendix 2
THE TRANSITION
STATE THEORY OF
ISOTOPE EFFECTS
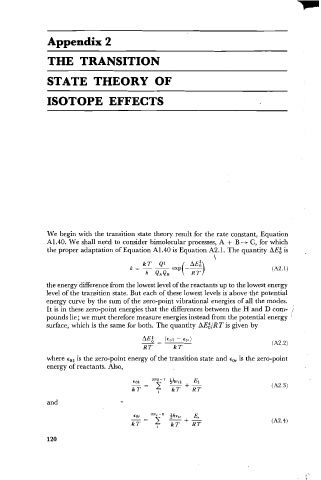
We begin with the transition state theory result for the rate constant, Equation
A1.40. We shall need to consider bimolecular processes, A + B -t C, for which
the proper adaptation of Equation A1.40 is Equation A2.1. The quantity AE; is .
\
the energy difference from the lowest level of the reactants up to the lowest energy
level of the transition state. But each of these lowest levels is above the potential
energy curve by the sum of the zero-point vibrational energies of all the modes.
It is in these zero-point energies that the differences between the H and D corn-
pounds lie; we must therefore measure energies instead from the potential energy '
surface, which is the same for both. The quantity AEA/RT is given by
where EO* is the zero-point energy of the transition state and E,, is the zero-point
energy of reactants. Also,
and