Page 44 - Modeling of Chemical Kinetics and Reactor Design
P. 44
14 Modeling of Chemical Kinetics and Reactor Design
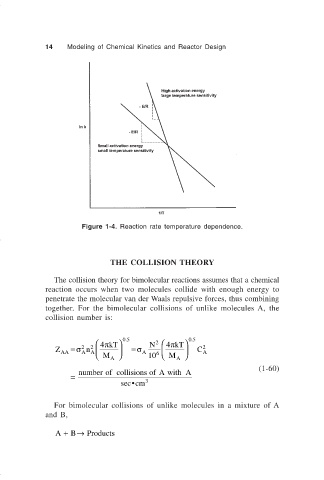
Figure 1-4. Reaction rate temperature dependence.
THE COLLISION THEORY
The collision theory for bimolecular reactions assumes that a chemical
reaction occurs when two molecules collide with enough energy to
penetrate the molecular van der Waals repulsive forces, thus combining
together. For the bimolecular collisions of unlike molecules A, the
collision number is:
π kT 05 . N π kT 05 .
2
4
4
2
Z AA =σ 2 A A =σ A C 2 A
n
M A 10 6 M A
(1-60)
= number of collisions of A with A
sec•cm 3
For bimolecular collisions of unlike molecules in a mixture of A
and B,
A + B→ Products