Page 336 - Modelling in Transport Phenomena A Conceptual Approach
P. 336
316 CHAPTER 8. STEADY MICROSCOPIC BALANCES WITHOUT GEN.
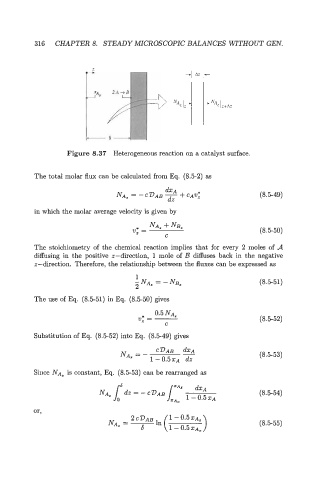
Figure 8.37 Heterogeneous reaction on a catalyst surface.
The total molar flux can be calculated from Eq. (8.52) as
(8.549)
in which the molar average velocity is given by
* NA, +NE,
v, = (8.550)
C
The stoichiometry of the chemical reaction implies that for every 2 moles of A
diffusing in the positive z-direction, 1 mole of B diffuses back in the negative
z-direction. Therefore, the relationship between the fluxes can be expressed as
1
- NA, = -NE, (8.551)
2
The use of Eq. (8.551) in Eq. (8.550) gives
0.5 NA,
v; = - (8.552)
C
Substitution of Eq. (8.552) into Eq. (8.549) gives
NA, = - C~AB ~XA (8.553)
1-0.5~~ dz
Since NA, is constant, Eq. (8.553) can be rearranged as
(8.554)
(8.555)