Page 20 - Modern Analytical Chemistry
P. 20
1400-CH01 9/9/99 2:20 PM Page 3
Chapter 1 Introduction 3
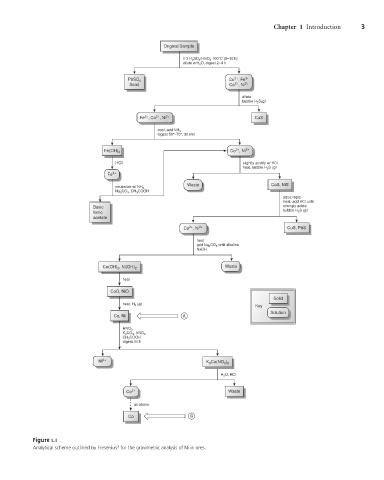
Original Sample
1:3 H 2 SO 4 /HNO 3 100°C (8–10 h)
dilute w/H 2 O, digest 2–4 h
2+
Cu , Fe 3+
PbSO 4
2+
Sand Co , Ni 2+
dilute
bubble H 2 S(g)
2+
3+
Fe , Co , Ni 2+ CuS
cool, add NH 3
digest 50°–70°, 30 min
2+
Co , Ni 2+
Fe(OH) 3
HCl slightly acidify w/ HCl
heat, bubble H 2 S (g)
Fe 3+
Waste CoS, NiS
neutralize w/ NH 3
Na 2 CO 3 , CH 3 COOH
aqua regia
heat, add HCl until
Basic strongly acidic
bubble H 2 S (g)
ferric
acetate
2+
Co , Ni 2+ CuS, PbS
heat
add Na 2 CO 3 until alkaline
NaOH
Waste
Co(OH) 2 , Ni(OH) 2
heat
CoO, NiO
Solid
heat, H 2 (g)
Key
Solution
Co, Ni A
HNO 3
K 2 CO 3 , KNO 3
CH 3 COOH
digest 24 h
Ni 2+ K 3 Co(NO 3 ) 5
H 2 O, HCl
Co 2+ Waste
as above
Co B
Figure 1.1
3
Analytical scheme outlined by Fresenius for the gravimetric analysis of Ni in ores.