Page 212 - Modern Analytical Chemistry
P. 212
1400-CH07 9/8/99 4:03 PM Page 195
Chapter 7 Obtaining and Preparing Samples for Analysis 195
7
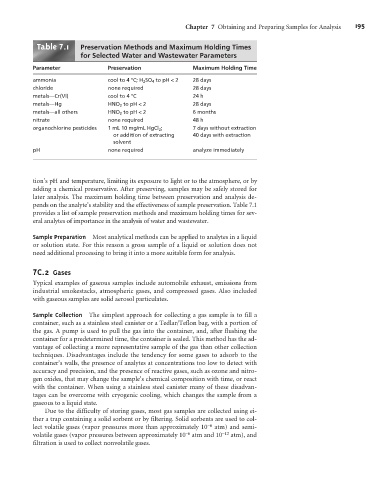
Table .1 Preservation Methods and Maximum Holding Times
for Selected Water and Wastewater Parameters
Parameter Preservation Maximum Holding Time
ammonia cool to 4 °C; H 2 SO 4 to pH < 2 28 days
chloride none required 28 days
metals—Cr(VI) cool to 4 °C 24 h
metals—Hg HNO 3 to pH < 2 28 days
metals—all others HNO 3 to pH < 2 6 months
nitrate none required 48 h
organochlorine pesticides 1 mL 10 mg/mL HgCl 2 ; 7 days without extraction
or addition of extracting 40 days with extraction
solvent
pH none required analyze immediately
tion’s pH and temperature, limiting its exposure to light or to the atmosphere, or by
adding a chemical preservative. After preserving, samples may be safely stored for
later analysis. The maximum holding time between preservation and analysis de-
pends on the analyte’s stability and the effectiveness of sample preservation. Table 7.1
provides a list of sample preservation methods and maximum holding times for sev-
eral analytes of importance in the analysis of water and wastewater.
Sample Preparation Most analytical methods can be applied to analytes in a liquid
or solution state. For this reason a gross sample of a liquid or solution does not
need additional processing to bring it into a more suitable form for analysis.
7C.2 Gases
Typical examples of gaseous samples include automobile exhaust, emissions from
industrial smokestacks, atmospheric gases, and compressed gases. Also included
with gaseous samples are solid aerosol particulates.
Sample Collection The simplest approach for collecting a gas sample is to fill a
container, such as a stainless steel canister or a Tedlar/Teflon bag, with a portion of
the gas. A pump is used to pull the gas into the container, and, after flushing the
container for a predetermined time, the container is sealed. This method has the ad-
vantage of collecting a more representative sample of the gas than other collection
techniques. Disadvantages include the tendency for some gases to adsorb to the
container’s walls, the presence of analytes at concentrations too low to detect with
accuracy and precision, and the presence of reactive gases, such as ozone and nitro-
gen oxides, that may change the sample’s chemical composition with time, or react
with the container. When using a stainless steel canister many of these disadvan-
tages can be overcome with cryogenic cooling, which changes the sample from a
gaseous to a liquid state.
Due to the difficulty of storing gases, most gas samples are collected using ei-
ther a trap containing a solid sorbent or by filtering. Solid sorbents are used to col-
lect volatile gases (vapor pressures more than approximately 10 –6 atm) and semi-
–6
volatile gases (vapor pressures between approximately 10 atm and 10 –12 atm), and
filtration is used to collect nonvolatile gases.