Page 187 - MODERN ELECTROCHEMISTRY
P. 187
126 CHAPTER 2
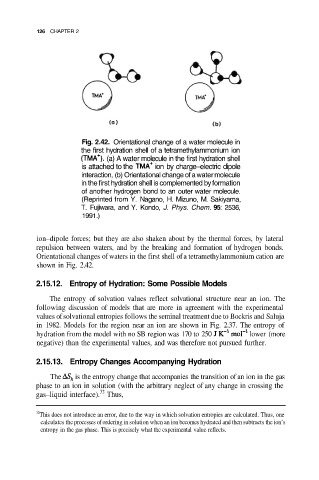
Fig. 2.42. Orientational change of a water molecule in
the first hydration shell of a tetramethylammonium ion
(a) A water molecule in the first hydration shell
is attached to the ion by charge–electric dipole
interaction, (b) Orientational change of a water molecule
in the first hydration shell is complemented by formation
of another hydrogen bond to an outer water molecule.
(Reprinted from Y. Nagano, H. Mizuno, M. Sakiyama,
T. Fujiwara, and Y. Kondo, J. Phys. Chem. 95: 2536,
1991.)
ion–dipole forces; but they are also shaken about by the thermal forces, by lateral
repulsion between waters, and by the breaking and formation of hydrogen bonds.
Orientational changes of waters in the first shell of a tetramethylammonium cation are
shown in Fig. 2.42.
2.15.12. Entropy of Hydration: Some Possible Models
The entropy of solvation values reflect solvational structure near an ion. The
following discussion of models that are more in agreement with the experimental
values of solvational entropies follows the seminal treatment due to Bockris and Saluja
in 1982. Models for the region near an ion are shown in Fig. 2.37. The entropy of
hydration from the model with no SB region was 170 to 250 lower (more
negative) than the experimental values, and was therefore not pursued further.
2.15.13. Entropy Changes Accompanying Hydration
The is the entropy change that accompanies the transition of an ion in the gas
phase to an ion in solution (with the arbitrary neglect of any change in crossing the
32
gas–liquid interface). Thus,
32
This does not introduce an error, due to the way in which solvation entropies are calculated. Thus, one
calculates the processes of ordering in solution when an ion becomes hydrated and then subtracts the ion’s
entropy in the gas phase. This is precisely what the experimental value reflects.