Page 306 - MODERN ELECTROCHEMISTRY
P. 306
242 CHAPTER 3
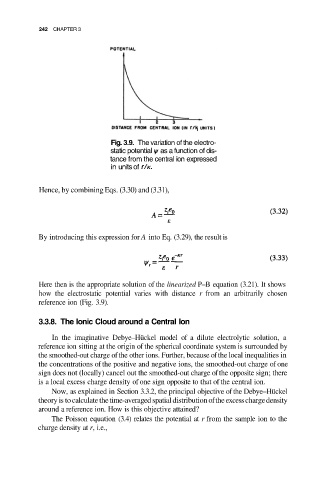
Fig. 3.9. The variation of the electro-
static potential as a function of dis-
tance from the central ion expressed
in units of
Hence, by combining Eqs. (3.30) and (3.31),
By introducing this expression for A into Eq. (3.29), the result is
Here then is the appropriate solution of the linearized P–B equation (3.21). It shows
how the electrostatic potential varies with distance r from an arbitrarily chosen
reference ion (Fig. 3.9).
3.3.8. The Ionic Cloud around a Central Ion
In the imaginative Debye–Hückel model of a dilute electrolytic solution, a
reference ion sitting at the origin of the spherical coordinate system is surrounded by
the smoothed-out charge of the other ions. Further, because of the local inequalities in
the concentrations of the positive and negative ions, the smoothed-out charge of one
sign does not (locally) cancel out the smoothed-out charge of the opposite sign; there
is a local excess charge density of one sign opposite to that of the central ion.
Now, as explained in Section 3.3.2, the principal objective of the Debye–Hückel
theory is to calculate the time-averaged spatial distribution of the excess charge density
around a reference ion. How is this objective attained?
The Poisson equation (3.4) relates the potential at r from the sample ion to the
charge density at r, i.e.,