Page 265 - Multifunctional Photocatalytic Materials for Energy
P. 265
248 Multifunctional Photocatalytic Materials for Energy
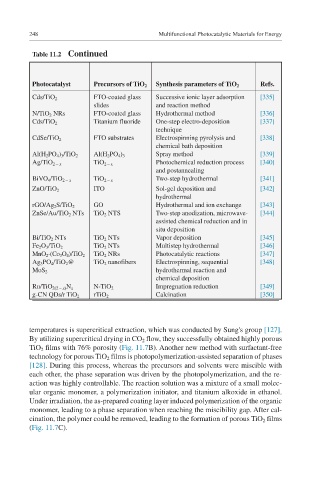
Table 11.2 Continued
Photocatalyst Precursors of TiO 2 Synthesis parameters of TiO 2 Refs.
Cds/TiO 2 FTO-coated glass Successive ionic layer adsorption [335]
slides and reaction method
N/TiO 2 NRs FTO-coated glass Hydrothermal method [336]
Cds/TiO 2 Titanium fluoride One-step electro-deposition [337]
technique
CdSe/TiO 2 FTO substrates Electrospinning pyrolysis and [338]
chemical bath deposition
Al(H 2 PO 4 ) 3 /TiO 2 Al(H 2 PO 4 ) 3 Spray method [339]
Ag/TiO 2 − x TiO 2 − x Photochemical reduction process [340]
and postannealing
BiVO 4 /TiO 2 − x TiO 2 − x Two-step hydrothermal [341]
ITO Sol-gel deposition and [342]
ZnO/TiO 2
hydrothermal
GO Hydrothermal and ion exchange [343]
rGO/Ag 2 S/TiO 2
ZnSe/Au/TiO 2 NTs TiO 2 NTS Two-step anodization, microwave- [344]
assisted chemical reduction and in
situ deposition
Bi/TiO 2 NTs TiO 2 NTs Vapor deposition [345]
TiO 2 NTs Multistep hydrothermal [346]
Fe 2 O 3 /TiO 2
TiO 2 NRs Photocatalytic reactions [347]
MnO 2 -(Co 3 O 4 )/TiO 2
Ag 3 PO 4 /TiO 2 @ TiO 2 nanofibers Electrospinning, sequential [348]
hydrothermal reaction and
MoS 2
chemical deposition
Impregnation reduction [349]
Ru/TiO 2(2 − x) N x N-TiO 2
Calcination [350]
g-CN QDs/r TiO 2 rTiO 2
temperatures is supercritical extraction, which was conducted by Sung's group [127].
By utilizing supercritical drying in CO 2 flow, they successfully obtained highly porous
TiO 2 films with 76% porosity (Fig. 11.7B). Another new method with surfactant-free
technology for porous TiO 2 films is photopolymerization-assisted separation of phases
[128]. During this process, whereas the precursors and solvents were miscible with
each other, the phase separation was driven by the photopolymerization, and the re-
action was highly controllable. The reaction solution was a mixture of a small molec-
ular organic monomer, a polymerization initiator, and titanium alkoxide in ethanol.
Under irradiation, the as-prepared coating layer induced polymerization of the organic
monomer, leading to a phase separation when reaching the miscibility gap. After cal-
cination, the polymer could be removed, leading to the formation of porous TiO 2 films
(Fig. 11.7C).