Page 39 - Book Hosokawa Nanoparticle Technology Handbook
P. 39
FUNDAMENTALS CH. 1 BASIC PROPERTIES AND MEASURING METHODS OF NANOPARTICLES
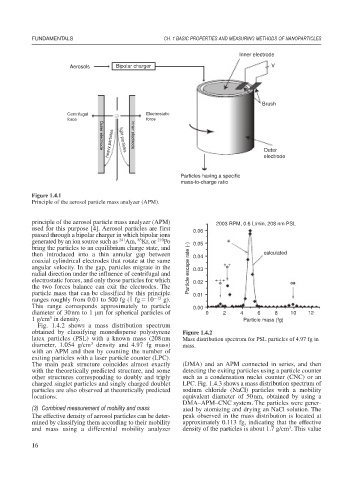
Inner electrode
Aerosols Bipolar charger V
Brush
Centrifugal Electrostatic
force force
heavy particles light particles Inner electrode Outer
Outer electrode
electrode
Particles having a specific
mass-to-charge ratio
Figure 1.4.1
Principle of the aerosol particle mass analyzer (APM).
principle of the aerosol particle mass analyzer (APM) 2003 RPM, 0.6 L/min, 208 nm PSL
used for this purpose [4]. Aerosol particles are first 0.06
passed through a bipolar charger in which bipolar ions
85
241
210
generated by an ion source such as Am, Kr, or Po 0.05 +
bring the particles to an equilibrium charge state, and
then introduced into a thin annular gap between 0.04 calculated
coaxial cylindrical electrodes that rotate at the same + +
angular velocity. In the gap, particles migrate in the Particle escape rate (-) 0.03
radial direction under the influence of centrifugal and
+
electrostatic forces, and only those particles for which 0.02 ++ +
the two forces balance can exit the electrodes. The
particle mass that can be classified by this principle 0.01
ranges roughly from 0.01 to 500 fg (1 fg 10 15 g).
This range corresponds approximately to particle 0.00
diameter of 30nm to 1 m for spherical particles of 0 2 4 6 8 10 12
3
1 g/cm in density. Particle mass (fg)
Fig. 1.4.2 shows a mass distribution spectrum
obtained by classifying monodisperse polystyrene Figure 1.4.2
latex particles (PSL) with a known mass (208nm Mass distribution spectrum for PSL particles of 4.97 fg in
3
diameter, 1.054 g/cm density and 4.97 fg mass) mass.
with an APM and then by counting the number of
exiting particles with a laser particle counter (LPC).
The main peak structure coincides almost exactly (DMA) and an APM connected in series, and then
with the theoretically predicted structure, and some detecting the exiting particles using a particle counter
other structures corresponding to doubly and triply such as a condensation nuclei counter (CNC) or an
charged singlet particles and singly charged doublet LPC. Fig. 1.4.3 shows a mass distribution spectrum of
particles are also observed at theoretically predicted sodium chloride (NaCl) particles with a mobility
locations. equivalent diameter of 50nm, obtained by using a
DMA–APM–CNC system. The particles were gener-
(3) Combined measurement of mobility and mass ated by atomizing and drying an NaCl solution. The
The effective density of aerosol particles can be deter- peak observed in the mass distribution is located at
mined by classifying them according to their mobility approximately 0.113 fg, indicating that the effective
3
and mass using a differential mobility analyzer density of the particles is about 1.7 g/cm . This value
16