Page 26 - Packed bed columns for absorption, desorption, rectification and direct heat transfer
P. 26
21
It is easy to see from Eq. (57) that the dimension of the diffusivity
The diffusivity characterizes the property of the substance to penetrate
in a given medium. Its value depends on the type of diffusing substance and the
medium in which the diffusion lakes place, on the temperature, pressure, and
concentrations. For the liquid phase especially for not very high pressures the
influence of the pressure is to be neglected.
According to the kinetic theory the diffusivity of the ideal gases is
s12
proportional to their viscosity and I and inversely proportional to the square
root of the molar mass. For real gases this dependence can be considered
2
5
4
approximate. Their diffusivity values varied from 10' to 10" m /s. The
s
4
diffusivity of liquid solutions is 10 - 10 times lower. It is also inversely
proportional to the viscosity.
Some simple methods for determination of the diffusivity in gas and
liquid phase taken from [40] are presented in the appendix of this chapter. Data
for determination of diffusivity in gases and liquids can be found in [52, 53] and
other reference books.
1.2.2.2. Diffusion in movable medium
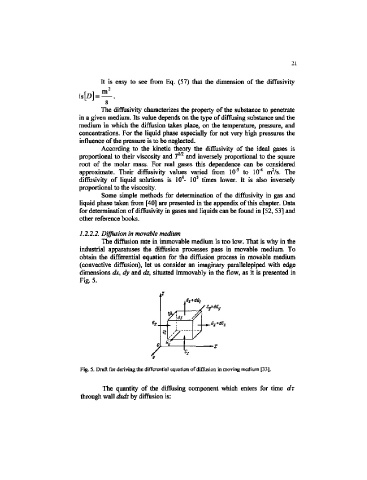
The diffusion rate in immovable medium is too low. That is why in the
industrial apparatuses the diffusion processes pass in movable medium. To
obtain the differential equation for the diffusion process in movable medium
(convective diffusion), let us consider an imaginary parallelepiped with edge
dimensions dx, dy and dz, situated immovably in the flow, as it is presented in
Fig. 5.
//
Fig. 5. Draft for deriving the differential equation of diffusion in moving medium [33],
The quantity of the diffusing component which enters for time dt
through wall dxdz by diffusion is: