Page 158 - Petroleum and Gas Field Processing
P. 158
The stability of oil–water emulsions could be viewed through the
following analysis. The relative difficulty of separating an emulsion into
two phases is a measure of its stability. A very stable emulsion is known as
a ‘‘tight’’ emulsion and its degree of stability is influenced by many factors.
Accordingly, we can best understand the resolution problem and, hence,
the treatment procedure if we consider the following factors:
1. Viscosity of oil: Separation is easier for a less viscous oil phase.
2. Density or gravity difference between oil and water phases: Bet-
ter separation is obtained for a larger difference.
3. Interfacial tension between the two phases (which is related to the
type of emulsifying agent): Separation is promoted if this force
is lowered (i.e., decreasing the interfacial tension).
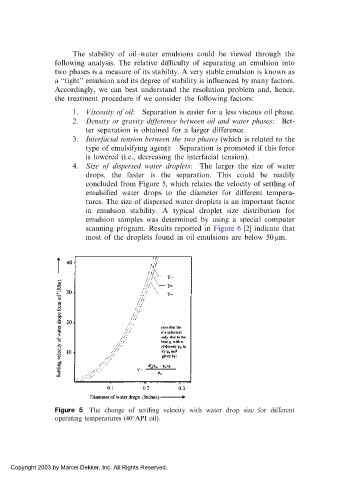
4. Size of dispersed water droplets: The larger the size of water
drops, the faster is the separation. This could be readily
concluded from Figure 5, which relates the velocity of settling of
emulsified water drops to the diameter for different tempera-
tures. The size of dispersed water droplets is an important factor
in emulsion stability. A typical droplet size distribution for
emulsion samples was determined by using a special computer
scanning program. Results reported in Figure 6 [2] indicate that
most of the droplets found in oil emulsions are below 50 mm.
Figure 5 The change of settling velocity with water drop size for different
operating temperatures (40 API oil).
Copyright 2003 by Marcel Dekker, Inc. All Rights Reserved.