Page 347 - Petrophysics 2E
P. 347
CAPILLARY PRESSURE 315
by forcing molecules from the interior into the surface. This indicates
that there is free energy associated with the surface that has the same
dimensions as the surface tension.
Capillary pressure is related to the curvature of the interface by the
expression developed by Plateau and applied to porous media by Leverett
[ 1,2]. Consider a segment of the interfacial surface separating two fluids
with a pressure difference across the interface, producing a curvilinear
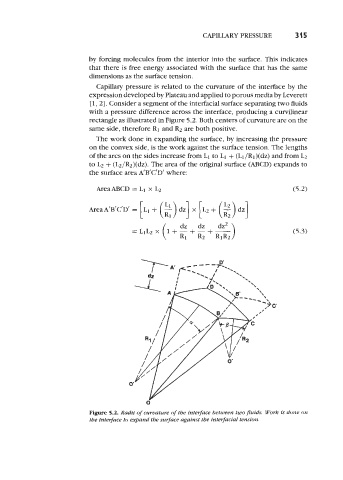
rectangle as illustrated in Figure 5.2. Both centers of curvature are on the
same side, therefore R1 and R2 are both positive.
The work done in expanding the surface, by increasing the pressure
on the convex side, is the work against the surface tension. The lengths
of the arcs on the sides increase from L1 to L1 + (LI/Rl)(dz) and from L2
to L2 + (Lz/Rz)(dz). The area of the original surface (ABCD) expands to
the surface area A’B’C’D’ where:
AreaABCD = L1 x Lz (5.2)
AreaA’B’C’D’ = [ L1 -t (2) dz] x [L2 + ($> dz]
(5.3)
0
Figure 5.2. Radii of curvature of tbe interface between twoJuids. Work is done on
the interjace to expand the surface against the interfacial tension.