Page 87 - Petrophysics 2E
P. 87
PROPERTIES OF SUBSURFACE FLUIDS 61
water as well as its history of contact with infiltrating waters. These
cations undergo reactions forming dolomite and enter into ion exchange
reactions; consequently, they are normally found in lower concentrations
than sodium cations. Other cations are present in concentrations less than
100 mg/L [13].
Oilfield waters are frequently referred to as connate or interstitial
water, which is found in small pores and between fine grains in water-wet
rocks. As defined by Collins, the two terms are synonymous and they
are indistinguishable as used in the petroleum literature [26]. “Connate”
implies that the water is the original fossil water present in the rocks
from the time of original deposition. One cannot be certain of this
because the original water may have been displaced or mixed with
other waters during the geologic history of the sedimentary formation.
Collins considers connate water as fossil water that has not been in
contact with water from other sources for a large part of its geologic
history.
Compressibility
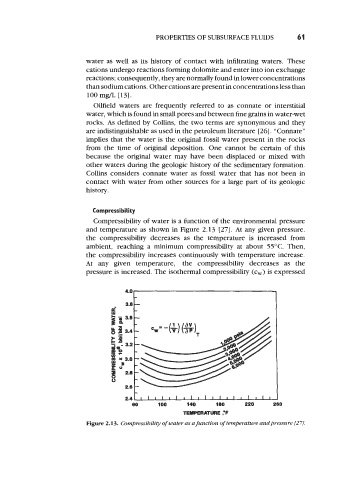
Compressibility of water is a function of the environmental pressure
and temperature as shown in Figure 2.13 [27]. At any given pressure,
the compressibility decreases as the temperature is increased from
ambient, reaching a minimum compressibility at about 55°C. Then,
the compressibility increases continuously with temperature increase.
At any given temperature, the compressibility decreases as the
pressure is increased. The isothermal compressibility (c,) is expressed
4.0 -
3.8 -
d -
2.6 -
0
-
2.4 1 I I 1 1 1 1 I 1 1 1 I 1 1 I I I I I
60 100 140 100 220 260
TEMPERATUIE :F
Figure 2.13. Compressibility of water as a function of temperature andpressure [27J