Page 102 - Petrophysics
P. 102
PETROLEUM CHEMISTRY 75
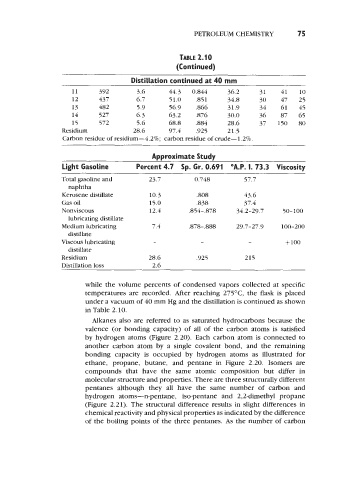
TABLE 2.1 0
(Continued)
Distibtion continued at 40 mm
11 392 3.6 44.3 0.844 36.2 31 41 10
12 437 6.7 51.0 ,851 34.8 30 47 25
13 482 5.9 56.9 366 31.9 34 61 45
14 527 6.3 63.2 .876 30.0 36 87 65
15 572 5.6 68.8 384 28.6 37 150 80
Residium 28.6 97.4 .925 21.5
Carbon residue of residium-4.2%; carbon residue of crude-l.2%.
Approximate Study
Light Gasoline Percent 4.7 Sp. Gr. 0.691 "A.P. 1. 73.3 Viscosity
Total gasoline and 23.7 0.748 57.7
naphtha
Kerosene distillate 10.3 ,808 43.6
Gas oil 15.0 .838 37.4
Nonviscous 12.4 ,854-,878 34.2-29.7 50-100
lubricating distillate
Medium lubricating 7.4 .878-. 888 29.7-27.9 100-200
distillate
Viscous lubricating - - - +lo0
distillate
Residium 28.6 ,925 215
Distillation loss 2.6
while the volume percents of condensed vapors collected at specific
temperatures are recorded. After reaching 275"C, the flask is placed
under a vacuum of 40 mm Hg and the distillation is continued as shown
in Table 2.10.
Alkanes also are referred to as saturated hydrocarbons because the
valence (or bonding capacity) of all of the carbon atoms is satisfied
by hydrogen atoms (Figure 2.20). Each carbon atom is connected to
another carbon atom by a single covalent bond, and the remaining
bonding capacity is occupied by hydrogen atoms as illustrated for
ethane, propane, butane, and pentane in Figure 2.20. Isomers are
compounds that have the same atomic composition but differ in
molecular structure and properties. There are three structurally different
pentanes although they all have the same number of carbon and
hydrogen atoms-n-pentane, iso-pentane and 2 ,Zdimethyl propane
(Figure 2.21). The structural difference results in slight differences in
chemical reactivity and physical properties as indicated by the difference
of the boiling points of the three pentanes. As the number of carbon