Page 220 - Photonics Essentials an introduction with experiments
P. 220
Optical Fibers and Optical Fiber Amplifiers
214 Advanced Topics
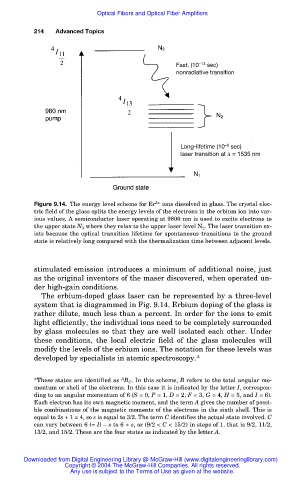
Fast, (10 –13 sec)
nonradiative transition
Long-lifetime (10 –9 sec)
laser transition at = 1535 nm
Figure 9.14. The energy level scheme for Er 3+ ions dissolved in glass. The crystal elec-
tric field of the glass splits the energy levels of the electrons in the erbium ion into var-
ious values. A semiconductor laser operating at 9800 nm is used to excite electrons to
the upper state N 3 where they relax to the upper laser level N 2 . The laser transition ex-
ists because the optical transition lifetime for spontaneous transitions to the ground
state is relatively long compared with the thermalization time between adjacent levels.
stimulated emission introduces a minimum of additional noise, just
as the original inventors of the maser discovered, when operated un-
der high-gain conditions.
The erbium-doped glass laser can be represented by a three-level
system that is diagrammed in Fig. 9.14. Erbium doping of the glass is
rather dilute, much less than a percent. In order for the ions to emit
light efficiently, the individual ions need to be completely surrounded
by glass molecules so that they are well isolated each other. Under
these conditions, the local electric field of the glass molecules will
modify the levels of the erbium ions. The notation for these levels was
developed by specialists in atomic spectroscopy.*
A
*These states are identified as B C . In this scheme, B refers to the total angular mo-
mentum or shell of the electrons. In this case it is indicated by the letter I, correspon-
ding to an angular momentum of 6 (S = 0, P = 1, D = 2, F = 3, G = 4, H = 5, and I = 6).
Each electron has its own magnetic moment, and the term A gives the number of possi-
ble combinations of the magnetic moments of the electrons in the sixth shell. This is
equal to 2s + 1 = 4, so s is equal to 3/2. The term C identifies the actual state involved. C
can vary between 6 (= I) – s to 6 + s, or (9/2 < C < 15/2) in steps of 1, that is 9/2, 11/2,
13/2, and 15/2. These are the four states as indicated by the letter A.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.