Page 35 - Photonics Essentials an introduction with experiments
P. 35
Electrons and Photons
Electrons and Photons 29
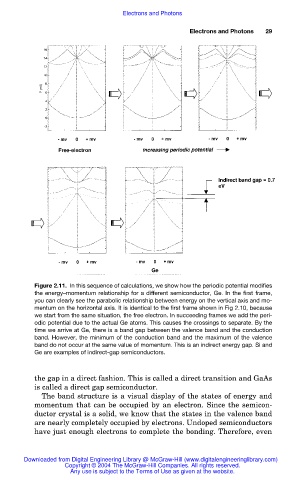
Figure 2.11. In this sequence of calculations, we show how the periodic potential modifies
the energy–momentum relationship for a different semiconductor, Ge. In the first frame,
you can clearly see the parabolic relationship between energy on the vertical axis and mo-
mentum on the horizontal axis. It is identical to the first frame shown in Fig 2.10, because
we start from the same situation, the free electron. In succeeding frames we add the peri-
odic potential due to the actual Ge atoms. This causes the crossings to separate. By the
time we arrive at Ge, there is a band gap between the valence band and the conduction
band. However, the minimum of the conduction band and the maximum of the valence
band do not occur at the same value of momentum. This is an indirect energy gap. Si and
Ge are examples of indirect-gap semiconductors.
the gap in a direct fashion. This is called a direct transition and GaAs
is called a direct gap semiconductor.
The band structure is a visual display of the states of energy and
momentum that can be occupied by an electron. Since the semicon-
ductor crystal is a solid, we know that the states in the valence band
are nearly completely occupied by electrons. Undoped semiconductors
have just enough electrons to complete the bonding. Therefore, even
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.