Page 117 - Physical chemistry understanding our chemical world
P. 117
84 ENERGY AND THE FIRST LAW OF THERMODYNAMICS
change in energy during vaporization can be symbolized as
We often omit the
small ‘m’; so, from U m (vaporization) . If we compare the molar energies for these two
now on, we assume similar processes, we see the following relation:
that changes in inter-
nal energy are molar
U m (condensation) =− U m (vaporization) (3.3)
quantities.
The two energies are equal and opposite because one process occurs
in the opposite direction to the other, yet the same amount of
Internal energy U is
a ‘state function’ be- material (and hence the same amount of energy) is involved in both.
Following from Equation (3.3), we say that internal energy is a
cause: (1) it is a ther-
modynamic property; state function. A more formal definition of state function is, ‘A ther-
and (2) its value modynamic property (such as internal energy) that depends only on
depends only on the the present state of the system, and is independent of its previous
present state of the history’. In other words, a ‘state function’ depends only on those
system, i.e. is indepen- variables that define the current state of the system, such as how
dent of the previous much material is present, whether it is a solid, liquid or gas, etc.
history. The concept of a state function can be quite difficult, so let us
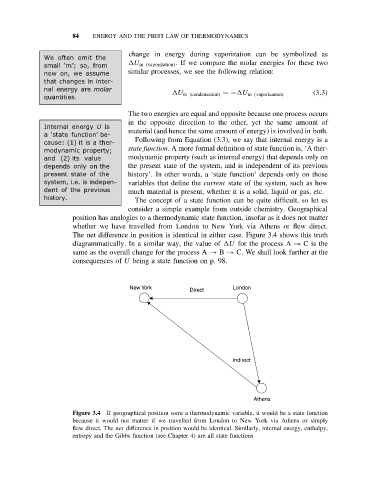
consider a simple example from outside chemistry. Geographical
position has analogies to a thermodynamic state function, insofar as it does not matter
whether we have travelled from London to New York via Athens or flew direct.
The net difference in position is identical in either case. Figure 3.4 shows this truth
diagrammatically. In a similar way, the value of U for the process A → Cisthe
same as the overall change for the process A → B → C. We shall look further at the
consequences of U being a state function on p. 98.
New York London
Direct
Indirect
Athens
Figure 3.4 If geographical position were a thermodynamic variable, it would be a state function
because it would not matter if we travelled from London to New York via Athens or simply
flew direct. The net difference in position would be identical. Similarly, internal energy, enthalpy,
entropy and the Gibbs function (see Chapter 4) are all state functions