Page 125 - Physical chemistry understanding our chemical world
P. 125
92 ENERGY AND THE FIRST LAW OF THERMODYNAMICS
The expression in Equation (3.6) is really a partial differential: the value of U depends
on both T and V , the values of which are connected via Equation (1.13). Accordingly,
we need to keep one variable constant if we are unambiguously to attribute changes in
C V to the other. The two subscript ‘V ’ terms tell us C is measured while maintaining
the volume constant. When the derivative is a partial derivative, it is usual to write
the ‘d’as‘∂’.
We call C V ‘the heat capacity at constant volume’. With the
We also call C V the volume constant, we measure C V without performing any work
isochoric heat capacity. (so w = 0), so we can write Equation (3.6) differently with dq
rather than dU.
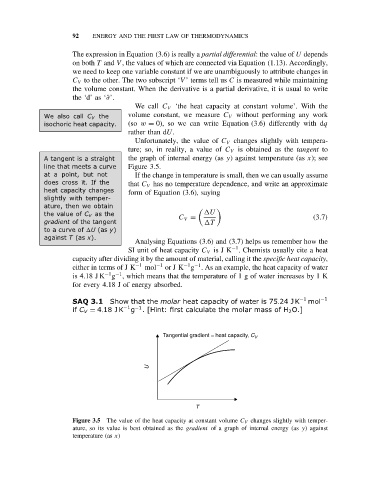
Unfortunately, the value of C V changes slightly with tempera-
ture; so, in reality, a value of C V is obtained as the tangent to
A tangent is a straight the graph of internal energy (as y) against temperature (as x); see
line that meets a curve Figure 3.5.
at a point, but not If the change in temperature is small, then we can usually assume
does cross it. If the that C V has no temperature dependence, and write an approximate
heat capacity changes form of Equation (3.6), saying
slightly with temper-
ature, then we obtain
the value of C V as the U
C V = (3.7)
gradient of the tangent T
to a curve of U (as y)
against T (as x).
Analysing Equations (3.6) and (3.7) helps us remember how the
−1
SI unit of heat capacity C V isJK . Chemists usually cite a heat
capacity after dividing it by the amount of material, calling it the specific heat capacity,
−1 −1
either in terms of J K −1 mol −1 or J K g . As an example, the heat capacity of water
−1 −1
is 4.18 J K g , which means that the temperature of 1 g of water increases by 1 K
for every 4.18 J of energy absorbed.
SAQ 3.1 Show that the molar heat capacity of water is 75.24 J K −1 mol −1
if C V = 4.18 J K −1 −1 . [Hint: first calculate the molar mass of H 2 O.]
g
Tangential gradient = heat capacity, C V
U
T
Figure 3.5 The value of the heat capacity at constant volume C V changes slightly with temper-
ature, so its value is best obtained as the gradient of a graph of internal energy (as y)against
temperature (as x)