Page 158 - Physical chemistry understanding our chemical world
P. 158
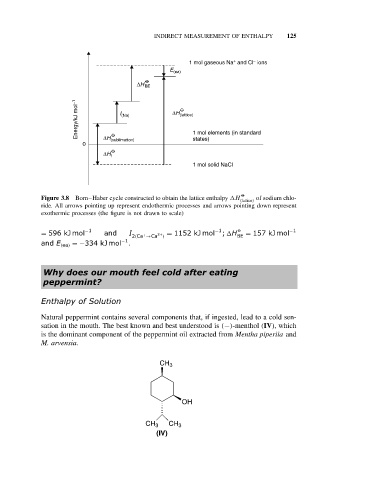
INDIRECT MEASUREMENT OF ENTHALPY 125
+ −
1 mol gaseous Na and Cl ions
E (ea)
O
∆H BE
Energy/kJ mol −1 I (Na) ∆H (lattice)
O
O
states)
0 ∆H (sublimation) 1 mol elements (in standard
O
∆H f
1 mol solid NaCl
O
Figure 3.8 Born–Haber cycle constructed to obtain the lattice enthalpy H of sodium chlo-
(lattice)
ride. All arrows pointing up represent endothermic processes and arrows pointing down represent
exothermic processes (the figure is not drawn to scale)
O
= 596 kJ mol −1 and I 2(Ca →Ca 2+ = 1152 kJ mol −1 ; H BE = 157 kJ mol −1
)
+
and E (ea) =−334 kJ mol −1 .
Why does our mouth feel cold after eating
peppermint?
Enthalpy of Solution
Natural peppermint contains several components that, if ingested, lead to a cold sen-
sation in the mouth. The best known and best understood is (−)-menthol (IV), which
is the dominant component of the peppermint oil extracted from Mentha piperiia and
M. arvensia.
CH 3
OH
CH 3 CH 3
(IV)