Page 181 - Physical chemistry understanding our chemical world
P. 181
148 REACTION SPONTANEITY AND THE DIRECTION OF THERMODYNAMIC CHANGE
the negative value of H overcomes the unfavourable positive − S term. In
fact, although the reaction is thermodynamically feasible, the rate of reaction (see
Chapter 8) is so small that we need to heat the reaction vessel strongly to about 550 K
to generate significant quantities of product to make the reaction viable.
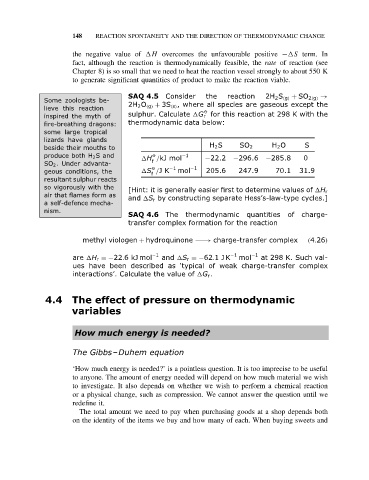
SAQ 4.5 Consider the reaction 2H 2 S (g) + SO 2(g) →
Some zoologists be- 2H 2 O (g) + 3S (s) , where all species are gaseous except the
lieve this reaction O
inspired the myth of sulphur. Calculate G r for this reaction at 298 K with the
fire-breathing dragons: thermodynamic data below:
some large tropical
lizards have glands
beside their mouths to H 2 S SO 2 H 2 O S
produce both H 2 Sand H /kJ mol −1 −22.2 −296.6 −285.8 0
O
SO 2 . Under advanta- f −1
O
geous conditions, the S /JK −1 mol 205.6 247.9 70.1 31.9
f
resultant sulphur reacts
so vigorously with the
[Hint: it is generally easier first to determine values of H r
air that flames form as and S r by constructing separate Hess’s-law-type cycles.]
a self-defence mecha-
nism.
SAQ 4.6 The thermodynamic quantities of charge-
transfer complex formation for the reaction
methyl viologen + hydroquinone −−−→ charge-transfer complex (4.26)
are H r =−22.6kJ mol −1 and S r =−62.1J K −1 mol −1 at 298 K. Such val-
ues have been described as ‘typical of weak charge-transfer complex
interactions’. Calculate the value of G r .
4.4 The effect of pressure on thermodynamic
variables
How much energy is needed?
The Gibbs–Duhem equation
‘How much energy is needed?’ is a pointless question. It is too imprecise to be useful
to anyone. The amount of energy needed will depend on how much material we wish
to investigate. It also depends on whether we wish to perform a chemical reaction
or a physical change, such as compression. We cannot answer the question until we
redefine it.
The total amount we need to pay when purchasing goods at a shop depends both
on the identity of the items we buy and how many of each. When buying sweets and