Page 355 - Physical Chemistry
P. 355
lev38627_ch11.qxd 3/14/08 1:10 PM Page 336
336
Chapter 11
Reaction Equilibrium where g(HOI) and a(H O) have each been taken as 1. Because of the extremely
2
in Nonideal Systems low value of K , the ionic strength is extremely low, and we can take g 1. If
a
we proceed as we did with HC H O , we have m(H O ) m(OI ) x and
2 3 2 3
m(HOI) 0.00010 mol/kg x. Since K is far less than the stoichiometric mo-
a
lality, we can set 0.00010 mol/kg x equal to 0.00010 mol/kg. We then have
2
2.3 10 11 mol/kg x /(0.00010 mol/kg), which gives x 4.8 10 8 mol/
kg m(H O ). However, this answer cannot be correct. We know that m(H O )
3 3
equals 1.0 10 7 mol/kg in pure water. A solution with m(H O ) 4.8 10 8
3
mol/kg would have a lower H O molality than pure water and would be basic.
3
However, HOI is an acid. The error here is failure to consider the contribution
to m(H O ) from the ionization (11.10) of water. In Examples 11.1 and 11.2, the
3
H O from the weak-acid ionization far exceeded that from the ionization of
3
water, but this is not true here. We must consider the two simultaneous equilib-
ria (11.17) and (11.10).
The ionization of water can be allowed for as follows. Let m be the stoi-
chiometric HOI molality, which is 1.0 10 4 mol/kg in this problem. As above,
we can approximate m(HOI) as m and g as 1. Equation (11.18) becomes K
a
m(H O )m(OI )/m. The electroneutrality condition is
3
m1H O 2 m1OH 2 m1OI 2 K >m1H O 2 m1OI 2
3
w
3
so m(OI ) m(H O ) K /m(H O ). Substitution of this m(OI ) expression
3
w
3
2
into K m(H O )m(OI )/m gives K m(H O ) /m K /m, so m(H O )
a 3 a 3 w 3
1/2
(K mK ) . Substitution of numerical values gives m(H O ) 1.1 10 7
w a 3
mol/kg. The solution is slightly acidic, as expected.
A systematic procedure for dealing with simultaneous equilibria is outlined
in Prob. 11.19.
Exercise
At what stoichiometric molality will a 25°C aqueous HOI solution have
m(H O ) 2.0 10 7 mol/kg? (Answer: 0.0013 mol/kg.)
3
For an aqueous solution of the weak acid HX with stoichiometric molality m, the
degree of dissociation a is defined as
m1X 2 m1X 2 1 1
a
m m1X 2 m1HX2 1 m1HX2>m1X 2 1 g m1H O 2>K a
2
3
where (11.15) was used. As m goes to zero, g goes to 1. Also, as m goes to zero, the
contribution of the ionization of HX to m(H O ) becomes negligible and all the H O
3 3
comes from the ionization of water. Hence in the limit of infinite dilution, m(H O )
3
becomes K 1/2 and the degree of dissociation of HX approaches
w
1 1
a at 25°C in H O (11.19)
q
2
1
7
1>2
1 K >K a 1 110 mol kg 2>K a
w
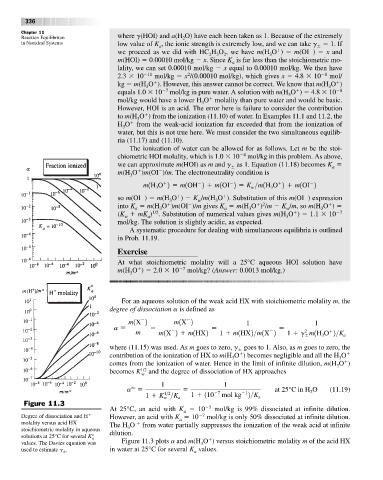
Figure 11.3
At 25°C, an acid with K 10 5 mol/kg is 99% dissociated at infinite dilution.
a
Degree of dissociation and H However, an acid with K 10 7 mol/kg is only 50% dissociated at infinite dilution.
a
molality versus acid HX The H O from water partially suppresses the ionization of the weak acid at infinite
stoichiometric molality in aqueous 3
solutions at 25°C for several K° a dilution.
values. The Davies equation was Figure 11.3 plots a and m(H O ) versus stoichiometric molality m of the acid HX
3
used to estimate g . in water at 25°C for several K values.
a