Page 89 - Physical Chemistry
P. 89
lev38627_ch02.qxd 2/29/08 3:11 PM Page 70
70
Chapter 2
The First Law of Thermodynamics
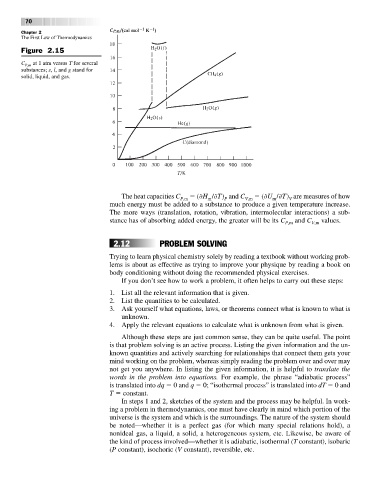
Figure 2.15
C P,m at 1 atm versus T for several
substances; s, l, and g stand for
solid, liquid, and gas.
The heat capacities C ( H / T) and C ( U / T) are measures of how
P,m m P V,m m V
much energy must be added to a substance to produce a given temperature increase.
The more ways (translation, rotation, vibration, intermolecular interactions) a sub-
stance has of absorbing added energy, the greater will be its C and C values.
P,m V,m
2.12 PROBLEM SOLVING
Trying to learn physical chemistry solely by reading a textbook without working prob-
lems is about as effective as trying to improve your physique by reading a book on
body conditioning without doing the recommended physical exercises.
If you don’t see how to work a problem, it often helps to carry out these steps:
1. List all the relevant information that is given.
2. List the quantities to be calculated.
3. Ask yourself what equations, laws, or theorems connect what is known to what is
unknown.
4. Apply the relevant equations to calculate what is unknown from what is given.
Although these steps are just common sense, they can be quite useful. The point
is that problem solving is an active process. Listing the given information and the un-
known quantities and actively searching for relationships that connect them gets your
mind working on the problem, whereas simply reading the problem over and over may
not get you anywhere. In listing the given information, it is helpful to translate the
words in the problem into equations. For example, the phrase “adiabatic process”
is translated into dq 0 and q 0; “isothermal process” is translated into dT 0 and
T constant.
In steps 1 and 2, sketches of the system and the process may be helpful. In work-
ing a problem in thermodynamics, one must have clearly in mind which portion of the
universe is the system and which is the surroundings. The nature of the system should
be noted—whether it is a perfect gas (for which many special relations hold), a
nonideal gas, a liquid, a solid, a heterogeneous system, etc. Likewise, be aware of
the kind of process involved—whether it is adiabatic, isothermal (T constant), isobaric
(P constant), isochoric (V constant), reversible, etc.