Page 288 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 288
258 Polymer-based Nanocomposites for Energy and Environmental Applications
Table 9.3 Comparison table among selected electrochemical energy
storage technologies [177]
Characteristics Capacitor Supercapacitors Battery
1
Specific energy (Wh kg ) <0.1 1–10 10–100
1
Specific power (Wkg ) ≫10,000 500–10,000 <1000
Discharge time 10 6 to 10 3 Seconds to minutes 0.3–3h
Charge time 10 6 to 10 3 Seconds to minutes 1–5h
Coulombic efficiency (%) About 100 85–98 70–85
Cycle life Almost infinite >500,000 About 1000
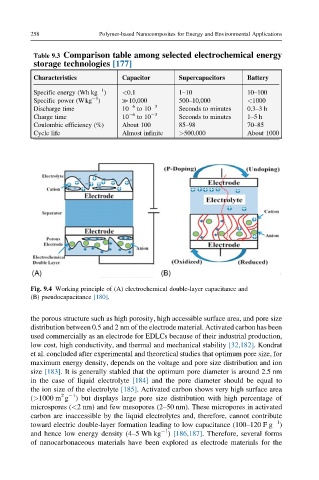
Fig. 9.4 Working principle of (A) electrochemical double-layer capacitance and
(B) pseudocapacitance [180].
the porous structure such as high porosity, high accessible surface area, and pore size
distribution between 0.5 and 2 nm of the electrode material. Activated carbon has been
used commercially as an electrode for EDLCs because of their industrial production,
low cost, high conductivity, and thermal and mechanical stability [32,182]. Kondrat
et al. concluded after experimental and theoretical studies that optimum pore size, for
maximum energy density, depends on the voltage and pore size distribution and ion
size [183]. It is generally stabled that the optimum pore diameter is around 2.5 nm
in the case of liquid electrolyte [184] and the pore diameter should be equal to
the ion size of the electrolyte [185]. Activated carbon shows very high surface area
2 1
(>1000 m g ) but displays large pore size distribution with high percentage of
microspores (<2 nm) and few mesopores (2–50 nm). These micropores in activated
carbon are inaccessible by the liquid electrolytes and, therefore, cannot contribute
1
toward electric double-layer formation leading to low capacitance (100–120 F g )
1
and hence low energy density (4–5Whkg ) [186,187]. Therefore, several forms
of nanocarbonaceous materials have been explored as electrode materials for the