Page 497 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 497
450 Polymer-based Nanocomposites for Energy and Environmental Applications
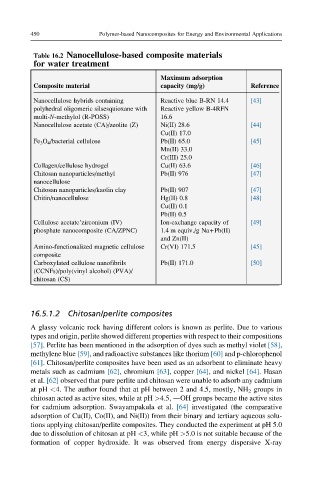
Table 16.2 Nanocellulose-based composite materials
for water treatment
Maximum adsorption
Composite material capacity (mg/g) Reference
Nanocellulose hybrids containing Reactive blue B-RN 14.4 [43]
polyhedral oligomeric silsesquioxane with Reactive yellow B-4RFN
multi-N-methylol (R-POSS) 16.6
Nanocellulose acetate (CA)/zeolite (Z) Ni(II) 28.6 [44]
Cu(II) 17.0
Fe 3 O 4 /bacterial cellulose Pb(II) 65.0 [45]
Mn(II) 33.0
Cr(III) 25.0
Collagen/cellulose hydrogel Cu(II) 63.6 [46]
Chitosan nanoparticles/methyl Pb(II) 976 [47]
nanocellulose
Chitosan nanoparticles/kaolin clay Pb(II) 907 [47]
Chitin/nanocellulose Hg(II) 0.8 [48]
Cu(II) 0.1
Pb(II) 0.5
Cellulose acetate’zirconium (IV) Ion-exchange capacity of [49]
phosphate nanocomposite (CA/ZPNC) 1.4 m equiv./g Na+Pb(II)
and Zn(II)
Amino-functionalized magnetic cellulose Cr(VI) 171.5 [45]
composite
Carboxylated cellulose nanofibrils Pb(II) 171.0 [50]
(CCNFs)/poly(vinyl alcohol) (PVA)/
chitosan (CS)
16.5.1.2 Chitosan/perlite composites
A glassy volcanic rock having different colors is known as perlite. Due to various
types and origin, perlite showed different properties with respect to their compositions
[57]. Perlite has been mentioned in the adsorption of dyes such as methyl violet [58],
methylene blue [59], and radioactive substances like thorium [60] and p-chlorophenol
[61]. Chitosan/perlite composites have been used as an adsorbent to eliminate heavy
metals such as cadmium [62], chromium [63], copper [64], and nickel [64]. Hasan
et al. [62] observed that pure perlite and chitosan were unable to adsorb any cadmium
at pH <4. The author found that at pH between 2 and 4.5, mostly, NH 2 groups in
chitosan acted as active sites, while at pH >4.5, dOH groups became the active sites
for cadmium adsorption. Swayampakula et al. [64] investigated (the comparative
adsorption of Cu(II), Co(II), and Ni(II)) from their binary and tertiary aqueous solu-
tions applying chitosan/perlite composites. They conducted the experiment at pH 5.0
due to dissolution of chitosan at pH <3, while pH >5.0 is not suitable because of the
formation of copper hydroxide. It was observed from energy dispersive X-ray