Page 354 - Pressure Swing Adsorption
P. 354
·ii L
330 PRESSURE SWING ADSORPTION
APPENDIX C 331
enrichment from atr (up to ahout 85% at nearly nil recovery), and for
nitrogen enrichment from aJr (up to 99% at nearly nil recovery). The latter
was the first kinetics-based PSA separation (in fact, the equilibnum se\ect1v-
1ty 1s m opposition to the kinetic seiect1v1ty), while the other applications he
cited expl01ted equilibrium select1vity. The adsorbents he c1·ted were silica gel
and activated alumina, zeoiite SA, and zeolite 4A, respectively. He showed
how hydrocarbons, including paraffins, 1soparaffins, olefins, di-oiefins, and
aromatics could be solit via three zeolites. He also explained high-pressure
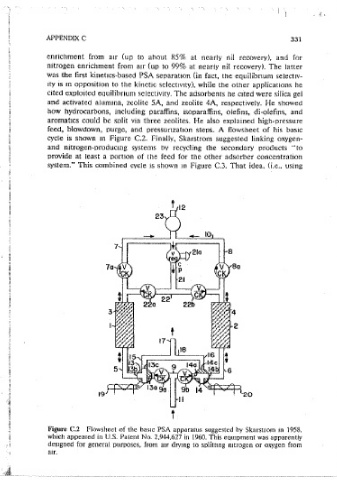
feed, blowdown, purge, and pressunzatmn steos. A flowsheet of his basic
cycle 1s shown m Figure C.2. Finally, Skarstrom suggested linking oxygen-
and nitrogen-oroducmg systems by recycling the secondaIY products ''to
provide at least a portion of the feed for the other adsorber concentration
system." This combined cycle is shown m Figure C.3. That idea. (i.e .. using
Figure C.t Flowshcct of the PSA apparatus suggeslcd by Perley m 1928, which
appeared m U.S. Patent No. 1,896,916 m 1933. This equipment was apparentlv
operated manµally, and was designed for splittmg hydrogen from water gas.
23
C.2.4 Erdmann
Another early patent that was essentially ignored was awarded to Erdmann ....... ,.._ 10
4
m 1941.', His process seemed to be a reduction to oractice of some of the 7
2
ideas suggested by Finlayson and Sharo and Perley; 10 Soecifically, he used
activated carbon · and a feed of 93% hydrogen and the balance carbon
monoxide to produce a pure product of 98 to 99% hydrogen, and an Ba
equ1molar byproduct. The operatmg temperature was - 50° C (to exploit
higher adsorption capacities at such a low temperature), and the pressure
range was apparently 1 to 0.13 atm.
3 4
C.2.5 Guerin de Montgareuil and Domme
2
A patent that' has received· some recognition w"s awarded in 19_'i7 to Gucnn
de Montgareuil -and Dominc. 15 It employed pressurization by feed, followed
by cocurrent blowdown, expioiting a type of kinetic effect, to yield the light
oroduct, and finally complete blowdown, yielding an enriched heavy compo-
nent. One advantage· it offered was s1molic1ty, ctue to the absence of flow
reversal, but that was met by the disadvantage of obtaining both products at
reiat1vcly low pressures. Despite that, 1t exploited, to a greater degree than
previous patents, the coupling of pressure and flow to achieve PSA separa- 19' 20
tions.
f
C.2.6 Skarstrom
Figure C.2 Flowsheet of the basic PSA apparatus suggested bv Skarstrom m 1958.
Skarstrom;s first PSA paten(~ was filed in 1958 and approved m 1960. He which appeared in U.S. Patent No. 2,944,627 in 1960. This eqmpment was apparentiy
described PSA systems for gas ctrymg and carbon dioxide removal, for oxygen designed for general purposes, from air drvmg to solittmg niirogen or oxygen from
air.