Page 108 - Primer on Enhanced Oil Recovery
P. 108
98 Primer on Enhanced Oil Recovery
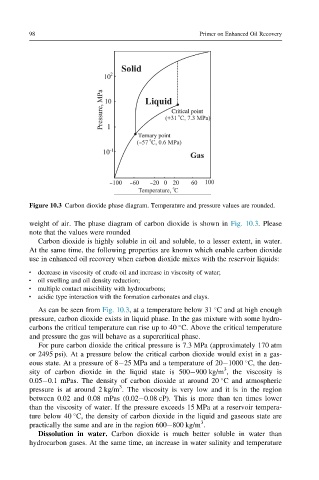
Figure 10.3 Carbon dioxide phase diagram. Temperature and pressure values are rounded.
weight of air. The phase diagram of carbon dioxide is shown in Fig. 10.3. Please
note that the values were rounded
Carbon dioxide is highly soluble in oil and soluble, to a lesser extent, in water.
At the same time, the following properties are known which enable carbon dioxide
use in enhanced oil recovery when carbon dioxide mixes with the reservoir liquids:
decrease in viscosity of crude oil and increase in viscosity of water;
oil swelling and oil density reduction;
multiple contact miscibility with hydrocarbons;
acidic type interaction with the formation carbonates and clays.
As can be seen from Fig. 10.3, at a temperature below 31 C and at high enough
pressure, carbon dioxide exists in liquid phase. In the gas mixture with some hydro-
carbons the critical temperature can rise up to 40 C. Above the critical temperature
and pressure the gas will behave as a supercritical phase.
For pure carbon dioxide the critical pressure is 7.3 MPa (approximately 170 atm
or 2495 psi). At a pressure below the critical carbon dioxide would exist in a gas-
eous state. At a pressure of 8 25 MPa and a temperature of 20 1000 C, the den-
3
sity of carbon dioxide in the liquid state is 500 900 kg/m , the viscosity is
0.05 0.1 mPas. The density of carbon dioxide at around 20 C and atmospheric
3
pressure is at around 2 kg/m . The viscosity is very low and it is in the region
between 0.02 and 0.08 mPas (0.02 0.08 cP). This is more than ten times lower
than the viscosity of water. If the pressure exceeds 15 MPa at a reservoir tempera-
ture below 40 C, the density of carbon dioxide in the liquid and gaseous state are
3
practically the same and are in the region 600 800 kg/m .
Dissolution in water. Carbon dioxide is much better soluble in water than
hydrocarbon gases. At the same time, an increase in water salinity and temperature