Page 41 - Primer on Enhanced Oil Recovery
P. 41
32 Primer on Enhanced Oil Recovery
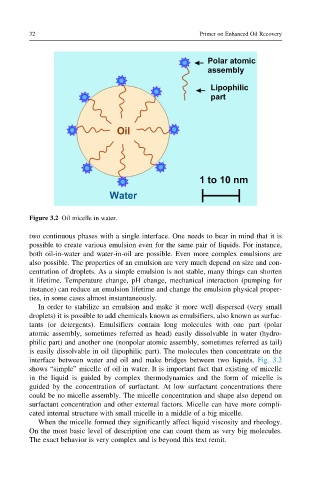
Figure 3.2 Oil micelle in water.
two continuous phases with a single interface. One needs to bear in mind that it is
possible to create various emulsion even for the same pair of liquids. For instance,
both oil-in-water and water-in-oil are possible. Even more complex emulsions are
also possible. The properties of an emulsion are very much depend on size and con-
centration of droplets. As a simple emulsion is not stable, many things can shorten
it lifetime. Temperature change, pH change, mechanical interaction (pumping for
instance) can reduce an emulsion lifetime and change the emulsion physical proper-
ties, in some cases almost instantaneously.
In order to stabilize an emulsion and make it more well dispersed (very small
droplets) it is possible to add chemicals known as emulsifiers, also known as surfac-
tants (or detergents). Emulsifiers contain long molecules with one part (polar
atomic assembly, sometimes referred as head) easily dissolvable in water (hydro-
philic part) and another one (nonpolar atomic assembly, sometimes referred as tail)
is easily dissolvable in oil (lipophilic part). The molecules then concentrate on the
interface between water and oil and make bridges between two liquids. Fig. 3.2
shows “simple” micelle of oil in water. It is important fact that existing of micelle
in the liquid is guided by complex thermodynamics and the form of micelle is
guided by the concentration of surfactant. At low surfactant concentrations there
could be no micelle assembly. The micelle concentration and shape also depend on
surfactant concentration and other external factors. Micelle can have more compli-
cated internal structure with small micelle in a middle of a big micelle.
When the micelle formed they significantly affect liquid viscosity and rheology.
On the most basic level of description one can count them as very big molecules.
The exact behavior is very complex and is beyond this text remit.